-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- BD® OMICS-One Protein Panels
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
-
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- BD® OMICS-One Protein Panels
- Japan (Japanese)
-
Change country/language
Old Browser
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Phosflow™ R718 Mouse Anti-p38 MAPK (pT180/pY182)
クローン 36/p38 (pT180/pY182) (RUO)
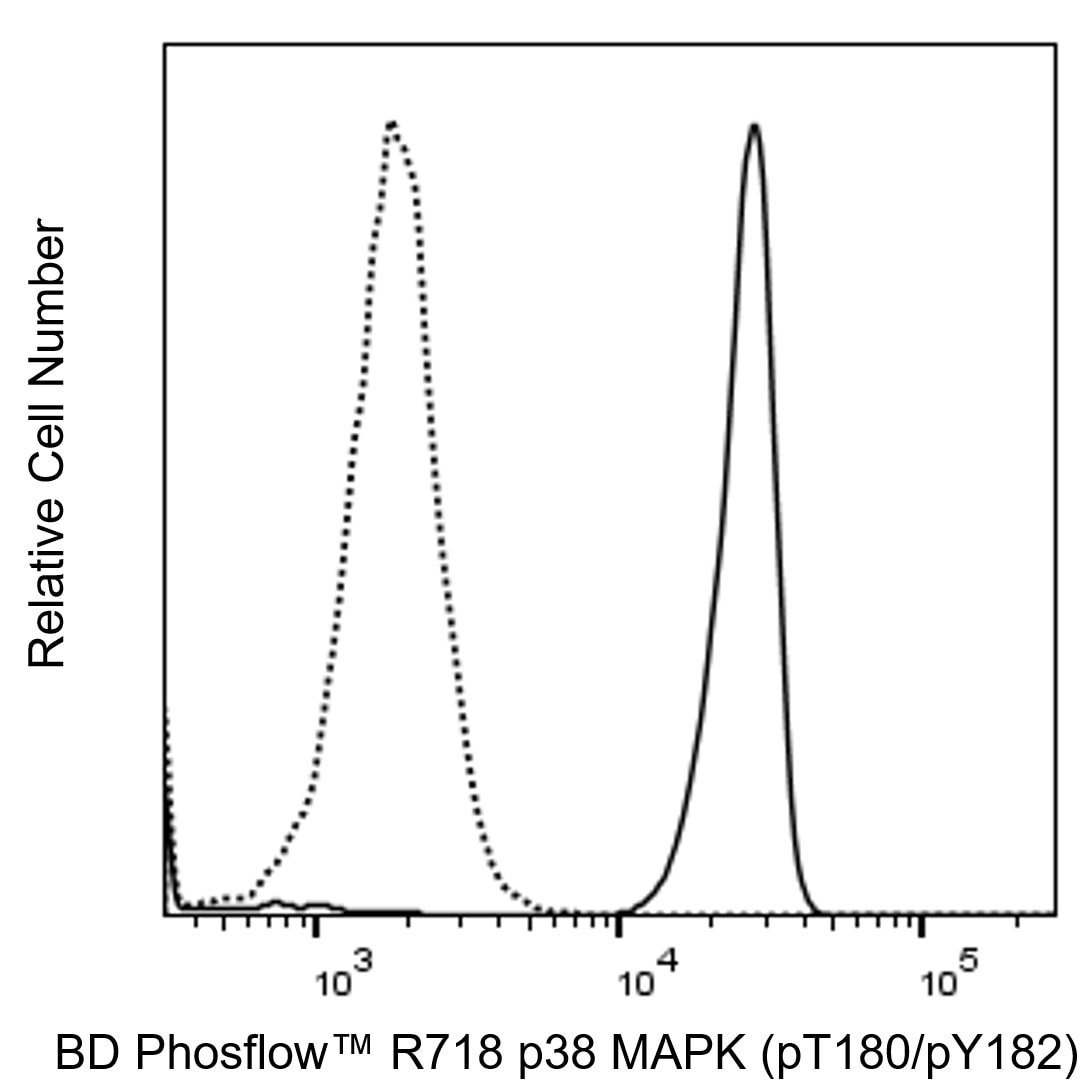
Flow cytometric analysis of p38 MAPK (pT180/pY182) expression in monocytes. Human peripheral blood mononuclear cells were either untreated (dashed line histogram) or treated (solid line histogram) by culture with 40 μM Anisomycin for 25 minutes at 37°C. The cells were fixed (10 min, 37°C) with BD Cytofix™ Fixation Buffer (Cat. No. 554655) and then permeabilized (on ice, 30 min) with BD Phosflow™ Perm Buffer III, Cat. No. 558050). Cells were washed twice in BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) and then stained with BD Phosflow™ R718 Mouse Anti-p38 MAPK (pT180/pY182) antibody (Cat. No. 563569). The fluorescence histograms showing the expressed levels of p38 MAPK (pT180/pY182) was derived from gated events with the forward and side light-scatter characteristics of intact monocytes. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software.

Flow cytometric analysis of p38 MAPK (pT180/pY182) expression in monocytes. Human peripheral blood mononuclear cells were either untreated (dashed line histogram) or treated (solid line histogram) by culture with 40 μM Anisomycin for 25 minutes at 37°C. The cells were fixed (10 min, 37°C) with BD Cytofix™ Fixation Buffer (Cat. No. 554655) and then permeabilized (on ice, 30 min) with BD Phosflow™ Perm Buffer III, Cat. No. 558050). Cells were washed twice in BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) and then stained with BD Phosflow™ R718 Mouse Anti-p38 MAPK (pT180/pY182) antibody (Cat. No. 563569). The fluorescence histograms showing the expressed levels of p38 MAPK (pT180/pY182) was derived from gated events with the forward and side light-scatter characteristics of intact monocytes. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software.

Flow cytometric analysis of p38 MAPK (pT180/pY182) expression in monocytes. Human peripheral blood mononuclear cells were either untreated (dashed line histogram) or treated (solid line histogram) by culture with 40 μM Anisomycin for 25 minutes at 37°C. The cells were fixed (10 min, 37°C) with BD Cytofix™ Fixation Buffer (Cat. No. 554655) and then permeabilized (on ice, 30 min) with BD Phosflow™ Perm Buffer III, Cat. No. 558050). Cells were washed twice in BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) and then stained with BD Phosflow™ R718 Mouse Anti-p38 MAPK (pT180/pY182) antibody (Cat. No. 563569). The fluorescence histograms showing the expressed levels of p38 MAPK (pT180/pY182) was derived from gated events with the forward and side light-scatter characteristics of intact monocytes. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software.


BD Phosflow™ R718 Mouse Anti-p38 MAPK (pT180/pY182)

BD Phosflow™ R718 Mouse Anti-p38 MAPK (pT180/pY182)
Regulatory Statusの凡例
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation and Storage
推奨アッセイ手順
BD™ CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to BD CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD CompBead to ensure that BD CompBeads are appropriate for your specific cellular application.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- This product is provided under an Agreement between BIOTIUM and BD Biosciences. This product, and only in the amount purchased by buyer, may be used solely for buyer’s own internal research, in a manner consistent with the accompanying product literature. No other right to use, sell or otherwise transfer (a) this product, or (b) its components is hereby granted expressly, by implication or by estoppel. This product is for research use only. Diagnostic uses require a separate license from Biotium, Inc. For information on purchasing a license to this product including for purposes other than research, contact Biotium, Inc., 3159 Corporate Place, Hayward, CA 94545, Tel: (510) 265-1027. Fax: (510) 265-1352. Email: btinfo@biotium.com.
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Alexa Fluor™ is a trademark of Life Technologies Corporation.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
関連製品




最近閲覧済み
Activation of the immune and inflammatory responses often involves the recognition of bacterial endotoxin (lipopolysaccharide or LPS). Binding of LPS by monocytes results in the production and release of proinflammatory cytokines, such as IL-1 and TNF. LPS-induced signaling cascades involve members of the Ser/Thr protein kinase family known as the Mitogen Activated Protein Kinases (MAPKs). MAPK signal transduction pathways mediate the effects of various extracellular stimuli on biological processes such as proliferation, differentiation, and death. The p38 MAPKs include p38α (MAPK14), β (MAPK11), γ (MAPK12), and δ (MAPK13). These Ser/Thr kinases are activated by dual phosphorylation on threonine (T) and tyrosine (Y) within the motif Thr-Gly-Tyr located in kinase subdomain VIII. Activation of p38 MAPK is mediated specifically by the MAP Kinase Kinases, MKK3, MKK4, and MKK6. This leads to the activation of multiple transcription factors (NF-κB, ATF-2, Elk-1, and CHOP) that induce expression of many different genes, including proinflammatory cytokine genes. Thus, p38 MAPKs are central kinases in multiple signal transduction pathways.
The 36/p38 (pT180/pY182) monoclonal antibody recognizes the conserved dual phosphorylated site pT180/pY182 of p38α, β, γ, and δ.
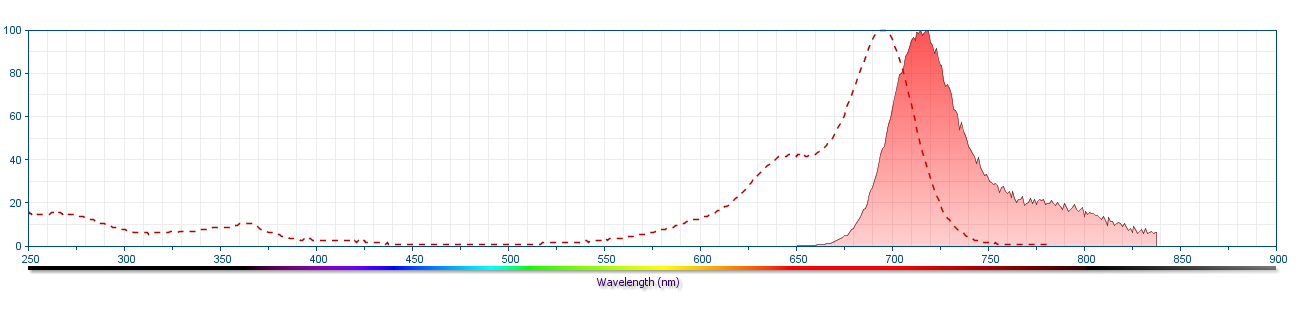
The antibody was conjugated to BD Horizon™ Red 718, which has been developed exclusively by for BD Biosciences as a better alternative to Alexa Fluor™ 700. BD Horizon™ Red 718 can be excited by the red laser (628 – 640 nm) and, with an Em Max around 718 nm, it can be detected using a 730/45 nm filter. Due to similar excitation and emission properties, we do not recommend using R718 in combination with APC-R700 or Alexa Fluor™ 700.
Development References (6)
-
Brunet A, Pouyssegur J. Identification of MAP kinase domains by redirecting stress signals into growth factor responses. Science. 1996; 272(5268):1652-1655. (Biology). View Reference
-
Franco LM, Gadkari M, Howe KN, et al. Immune regulation by glucocorticoids can be linked to cell type-dependent transcriptional responses.. J Exp Med. 2019; 216(2):384-406. (Clone-specific: Flow cytometry). View Reference
-
Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994; 265(5173):808-811. (Biology). View Reference
-
Perez OD, Mitchell D, Campos R, Gao GJ, Li L, Nolan GP. Multiparameter analysis of intracellular phosphoepitopes in immunophenotyped cell populations by flow cytometry. Curr Protoc Cytom. 2005; 6.20.1-6.20.22. (Clone-specific: Flow cytometry). View Reference
-
Suni MA, Maino VC. Flow cytometric analysis of cell signaling proteins. Methods Mol Biol. 2011; 717:155-169. (Clone-specific: Flow cytometry). View Reference
-
Winston BW, Chan ED, Johnson GL, Riches DW. Activation of p38mapk, MKK3, and MKK4 by TNF-alpha in mouse bone marrow-derived macrophages. J Immunol. 1997; 159(9):4491-4497. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
