-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- BD® OMICS-One Protein Panels
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Immune Profiler Protein Panel
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
BD OptEIA™ Mouse IgG2a ELISA Set
クローン B27 (RUO)


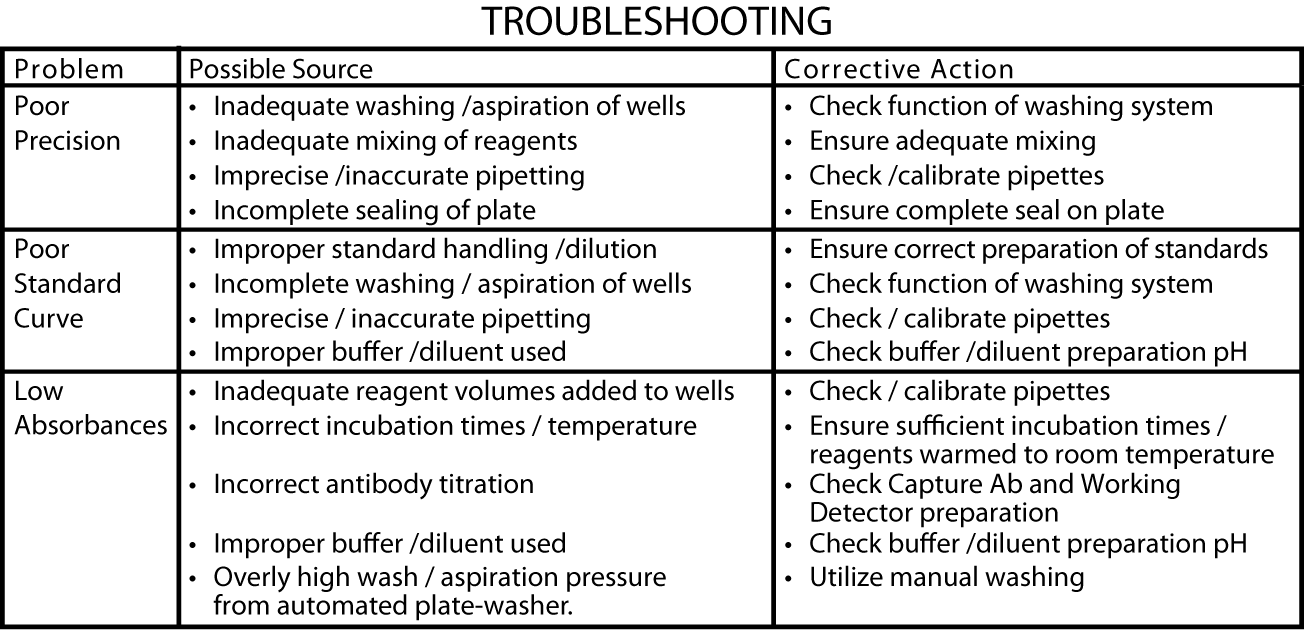


This standard curve is fordemonstration only. A standard curve must be run with each assay. "Typical Standard Curve" and 20-plate yield were obtained in the BD Biosciences Pharmingen laboratory, using the recommended procedure and manual plate washing.







Regulatory Statusの凡例
Becton, Dickinson and Companyの書面による明示的な許諾を得た使用以外での製品の使用は固く禁じられています。
説明
The OptEIA™ Set for mouse IgG2a contains the components necessary to develop enzyme-linked immunosorbent assays (ELISA) for natural or purified mouse IgG2a in serum, plasma, and cell culture supernatants. Sufficient materials are provided to yield approximately 20 plates of 96-wells if the recommended storage, materials, buffer preparation, and assay procedure are followed as specified in this package.
Assay Optimization
BD OptEIA™ Sets allow flexible assay design to fit individual laboratory needs. To design an immunoassay with different sensitivity and dynamic range, the following parameters can be varied: Capture, Detection Antibody titers, Incubation time, Incubation temperature, Assay Diluent formulation, Buffer pH, ionic strength, protein concentration, Type of substrate, Washing technique (i.e., number of wash repetitions and soak times).
調製と保管
推奨アッセイ手順
Recommended buffers, solutions
Note: Do not use sodium azide in these preparations. Sodium azide inactivates the horseradish peroxidase enzyme.
The BD OptEIA™ Reagent Set B (Cat. No 550534) containing Coating Buffer, Assay Diluent, Substrate Reagents A and B, Stop Solution and 20X Wash Buffer Concentrate is recommended.
1. Coating Buffer - 0.1 M Sodium Carbonate, pH 9.5; 7.13 g NaHCO3, 1.59 g Na2CO3; q.s. to 1.0 L; pH to 9.5 with 10 N NaOH. Freshly prepare or use within 7 days of preparation, stored at 2-8°C.
2. Assay Diluent- PBS* with 10% FBS#, pH 7.0. The BD Pharmingen™ Assay Diluent (Cat. No. 555213) is recommended.
*Phosphate-Buffered Saline: 80.0 g NaCl, 11.6 g Na2HPO4, 2.0 g KH2PO4, 2.0 g KCL, q.s. to 10 L; pH to 7.0.
#Fetal Bovine Serum: Hyclone Cat. No. SH30088 (heatinactivated) recommended.
Freshly prepare or use within 3 days of preparation, with 2-8°C storage.
3. Wash Buffer - PBS* with 0.05% Tween-20. Freshly prepare or use within 3 days of preparation, stored at 2-8°C.
4. Substrate Solution - Tetramethylbenzidine (TMB) and Hydrogen Peroxide. The BD Pharmingen™ TMB Substrate Reagent Set (Cat. No. 555214) is recommended.
5. Stop Solution - 1 M H3PO4 or 2 N H2SO4
Additional Materials Required
1. 96-well BD Falcon™ ELISA plates (Cat. No. 353279) are recommended
2. Microplate reader capable of measuring absorbance at 450 nm
3. Precision pipettes
4. Graduated cylinder, one liter
5. Deionized or distilled water
6. Wash bottle or automated washer
7. Log-log graph paper or automated data reduction
8. Tubes to prepare standard dilutions
9. Laboratory timer
10. Plate sealers or parafilm
Specimen Collection and Handling
Specimens should be clear, non-hemolyzed and non-lipemic.
Cell culture supernatants: Remove any particulate material by centrifugation and assay immediately or store samples at ≤ -20°C. Avoid repeated freeze-thaw cycles.
Serum: Use a serum separator tube and allow samples to clot for30 minutes, then centrifuge for 10 minutes at 1000 x g. Remove serum and assay immediately or store samples at ≤ -20° C. Avoid repeated freeze-thaw cycles.
Plasma: Collect plasma using citrate, EDTA, or heparin as anticoagulant. Centrifuge for 10 minutes at 1000 x g within 30 minutes of collection. Assay immediately or store samples at ≤ -20° C. Avoid repeated freeze-thaw cycles.
Standards Preparation and Handling
1. Reconstitution: After warming lyophilized standard to room temperature, carefully open vial to avoid loss of material. Reconstitute lyophilized standard with 1.0 mL of deionized water to yield a stock standard. Allow the standard to equilibrate for at least 15 minutes before making dilutions. Vortex gently to mix.
2. Storage/ handling of reconstituted standard: After reconstitution, immediately aliquot standard stock in polypropylene vials at 50 μl per vial and freeze at -80°C for up to 6 months. If necessary, store at 2-8° C for up to 8 hours prior to aliquotting/freezing. Do not leave reconstituted standard at room temperature.
3. Standards Preparation for Assay:
a. Prepare a 200 ng/mL standard from the stock standard. Vortex to mix. (See dilution instructions on Instruction/Analysis Certificate.)
b. Add 300 μL Assay Diluent to 6 tubes. Label as 100 ng/mL, 50 ng/mL, 25 ng/mL, 12.5 ng/mL, 6.3 ng/mL, and 3.1 ng/mL.
c. Perform serial dilutions by adding 300 μL of each standard to the next tube and vortexing between each transfer. Assay Diluent serves as the zero standard (0 pg/mL).
Standardization: This immunoassay is calibrated against purified mouse IgG2a.
Detailed Assay Procedure
1. Coat microwells with 100 μL per well of Capture Antibody diluted in Coating Buffer. For recommended antibody coating dilution, see lot-specific Instruction/Analysis Certificate. Seal plate and incubate overnight at 4° C.
2. Aspirate wells and wash 3 times with ≥ 300 μL/well Wash Buffer. After last wash, invert plate and blot on absorbent paper to remove any residual buffer.
3. Block plates with ≥ 200 μL/well Assay Diluent. Incubate at RT for 1 hour.
4. Aspirate/wash as in step 2.
5. Prepare standard and sample dilutions in Assay Diluent. See "Standards Preparation and Handling."
6. Pipette 100 μL of each standard, sample, and control into appropriate wells. Seal plate and incubate for 2 hours at RT.
7. Aspirate/ wash as in step 2, but with 5 total washes.
8. Add 100 μL of Detection Antibody diluted in Assay Diluent to each well. Seal plate and incubate for 1 hour at RT.
9. Aspirate/ wash as in step 2, but with 5 total washes.
10. Add 100 μL of Enzyme Reagent diluted in Assay Diluent to each well. Seal plate and incubate for 30 min at RT.
11. Aspirate/ wash as in step 2, but with 7 total washes.
NOTE: In this final wash step, soak wells in wash buffer for 30 seconds to 1 minute for each wash.
12. Add 100 μL of Substrate Solution to each well. Incubate plate (without plate sealer) for 30 minutes at room temperature in the dark.
13. Add 50 μL of Stop Solution to each well.
14. Read absorbance at 450 nm within 30 minutes of stopping reaction. If wavelength correction is available, subtract absorbance at 570 nm from absorbance 450 nm.
Assay Procedure Summary
1. Add 100 μL diluted Capture Ab to each well. Incubate overnight at 4°C.
2. Aspirate and wash 3 times.
3. Block plates: 200 μL Assay Diluent to each well. Incubate 1 hr RT
4. Aspirate and wash 3 times.
5. Add 100 μL standard or sample to each well. Incubate 2 hr RT.
6. Aspirate and wash 5 times.
7. Add 100 μL diluted Detection Ab to each well. Incubate 1 hr RT.
8. Aspirate and wash 5 times.
9. Add 100 μL diluted SAv-HRP to each well. Incubate 30 min RT.
10. Aspirate and wash 7 times (with 30 sec to 1 min soaks)
11. Add 100 μL Substrate Solution to each well. Incubate 30 min RT in dark
12. Add 50 μL Stop Solution to each well. Read at 450 nm within 30 min with λ correction 570 nm.
Calculation of Results
Calculate the mean absorbance for each set of duplicate standards, controls and samples. Subtract the mean zero standard absorbance from each.
Plot the standard curve on log-log graph paper, with IgG2a concentration on the x-axis and absorbance on the y-axis. Draw the best fit curve through the standard points.
To determine the IgG2a concentration of the unknowns, find the unknown's mean absorbance value on the y-axis and draw a horizontal line to the standard curve. At the point of intersection, draw a vertical line to the x-axis and read the IgG2a concentration. If samples were diluted, multiply the IgG2a concentration by the dilution factor.
Computer data reduction may also be employed, utilizing log-log regression analysis.
Specificity
Cross Reactivity: The following immunoglobulins were tested at ≥ 10 µg/ml for potential cross-reactivity in the BD OptEIA™ mouse IgG2a ELISA Set. No significant cross-reactivities were identified (values ≥ 3.1 ng/ml).
Mouse Immunoglobulins: IgG1 (κ), IgG1 (λ), IgG2b (κ), IgG2b (λ), IgG3 (κ), IgG3 (λ), IgM (κ), IgM (λ), IgA (κ), IgA (λ), IgE (κ)
Rat Immunoglobulins: IgG1 (κ), IgG1 (λ), IgG2a (κ), IgG2a (λ), IgG2b (κ), IgG2b (λ),IgG2c (κ), IgM (κ), IgM (λ), IgA (κ), IgE (κ)
Serum samples from different mouse species (BALB/c, NZB, NZBWF1,and BXSB), immunized or unimmunized, were tested in this assay. Themeasured IgG2a concentrations ranged from 0.16 mg/ml to 5.46 mg/ml. No detectable IgG2a was found in SCID mouse (RAG-/-) serum.
Immunoglobulin Allotypes
Researchers should be aware that slight differences with the measurements of IgG2a levels may occur across different species and/or allotypes.
The BD OptEIA™ mouse IgG2a Set is known to have different affinities for IgG2a from mice of the b allotype as compared with the a allotype. It recognizes a allotype IgG2a approximately 8.1 time better than b allotype IgG2a. Because a purified monoclonal IgG2a antibody of the a allotype is used for the standard in this assay, a conversion factor of 8.1 should be used to quantitate b allotype IgG2a levels. For example, a group of C57BL/6 (b allotype) mouse serum samples were tested using this assay and showed a concentration range of 1.04-7.84 µg/ml, the actual range should be of 8.4-63.5 µg/ml after the conversion.
Limitations of the Procedure
· Samples that generate absorbance values higher than the standard curve should be diluted with Standard Diluent and re-assayed.
· Interference by drug metabolites, soluble receptors, or other binding proteins in specimens has not been thoroughly investigated. The possibility of interference cannot be excluded.
· BD OptEIA™ Sets are intended for use as an integral unit. Do not mix reagents from different Set batches. Reagents from other manufacturers are not recommended for use in this Set.
製品通知
- For online training for BD OptEIA™ Set ELISA Techniques, please refer to http://www.bdbiosciences.com/OptEIA/downloads.shtml
- Samples that generate absorbance values higher than the standard curve should be diluted with Standard Diluent and re-assayed.
- Interference by drug metabolites, soluble receptors, or other binding proteins in specimens has not been thoroughly investigated. The possibility of interference cannot be excluded.
- BD OptEIA™ Sets are intended for use as an integral unit. Do not mix reagents from different Set batches. Reagents from other manufacturers are not recommended for use in this Set.
- Reagents which contain preservatives may be toxic if ingested, inhaled, or in contact with skin.
- Handle all serum and plasma specimens in accordance with NCCLS guidelines for preventing transmission of blood-borne infections.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- ProClin is a trademark of Rohm and Haas Company.
| 説明 | 数量/容量 | Part Number | EntrezGene ID |
|---|---|---|---|
| N/A | null | N/A | N/A |
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.