-
Reagents
- Flow Cytometry Reagents
-
蛋白质印迹试剂
- 免疫分析 试剂
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ 附件试剂盒
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
显微成像试剂
-
Cell Preparation and Separation Reagents
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location or be switched to your location?

BD OneFlow™
B-cell Chronic Lymphoproliferative Diseases Tube 1
(B-CLPD T1)
In combination with the BD OneFlow™ LST, it is intended as an aid in the diagnosis of chronic lymphocytic leukemia (CLL) and other B cell chronic lymphoproliferative diseases.
Overview
The BD OneFlow™ B-CLPD T1 is part of the innovative BD OneFlow™ Solution, a comprehensive set of reagents, setup beads, protocols and assay templates to reproducibly set up the flow cytometer and stain, acquire and analyze patient specimens for immunophenotyping of normal and aberrant cell populations. It is built on the research and validation work of the EuroFlow™ Consortium on the characterization of hematological malignancies for improved accurate diagnosis.1*
The BD OneFlow™ B-CLPD T1 is intended for flow cytometric immunophenotyping of B cells in peripheral blood and bone marrow as an aid in the diagnosis of chronic lymphocytic leukemia (CLL) and other B cell chronic lymphoproliferative diseases.
Other reagents belonging to the BD OneFlow™ Solution are BD OneFlow™ Lymphoid Screening Tube (LST), BD OneFlow™ Plasma Cell Screening Tube (PCST), BD OneFlow™ Plasma Cell Disorders (PCD) Tube and BD OneFlow™ Acute Leukemia Orientation Tube (ALOT).
View video describing the BD OneFlow™ Solution on the BD FACSLyric™ Flow Cytometer.
View videos below describing how standardization, efficiency, accuracy and reproducibility can be achieved using the BD OneFlow™ Solution on the BD FACSCanto™ II Flow Cytometer.
FEATURES
The BD OneFlow™ B-CLPD T1:

- Is a preconfigured single-use, ready-to-use 8-color reagent provided in a single tube
- Is a classification tube that is used for specimens with B lineage populations needing further investigation in combination with the BD OneFlow™ Lymphoid Screening Tube (LST)
- Is available in the 20 test/box size (4 pouches of 5 tubes each)
- Allows for reagent visual identification with boxes, pouches and tubes color coded with a lighter blue color than the one identifying BD OneFlow™ LST. The blue color (dark and light) identifies the BD OneFlow™ B-cell Chronic Lymphoproliferative Disease Panel
The BD OneFlow™ Reagents increase efficiency by simplifying instrument standardization, reducing the time to prepare the instrument as well as the technical burden and training needs.2
For BD FACSLyric™ Flow Cytometer users:
- BD® CS&T IVD Beads standardize setup and monitoring for consistent performance and ensure reproducibility through Universal Setup
- BD® FC Beads (7-Color, 5-Color and 2-Color Kits) support consistency of results, eliminating the need for using cells for compensation
- BD® CS&T Beads and BD® FC Beads kits necessary for instrument setup and compensation can be used for other CE-IVD assays on the BD FACSLyric™ Flow Cytometer
For BD FACSCanto™ II Flow Cytometer users:
- BD FACSDiva™ CS&T IVD Beads standardize setup and monitoring for consistent performance and ensure reproducibility with Application Settings
- BD OneFlow™ Setup Beads ensure data accuracy and reproducibility by providing assay-specific target values, as per EuroFlow™ SOPs.3
- Specifically designed BD® FC Beads eliminate the need for using single-vial reagents and cells for compensation
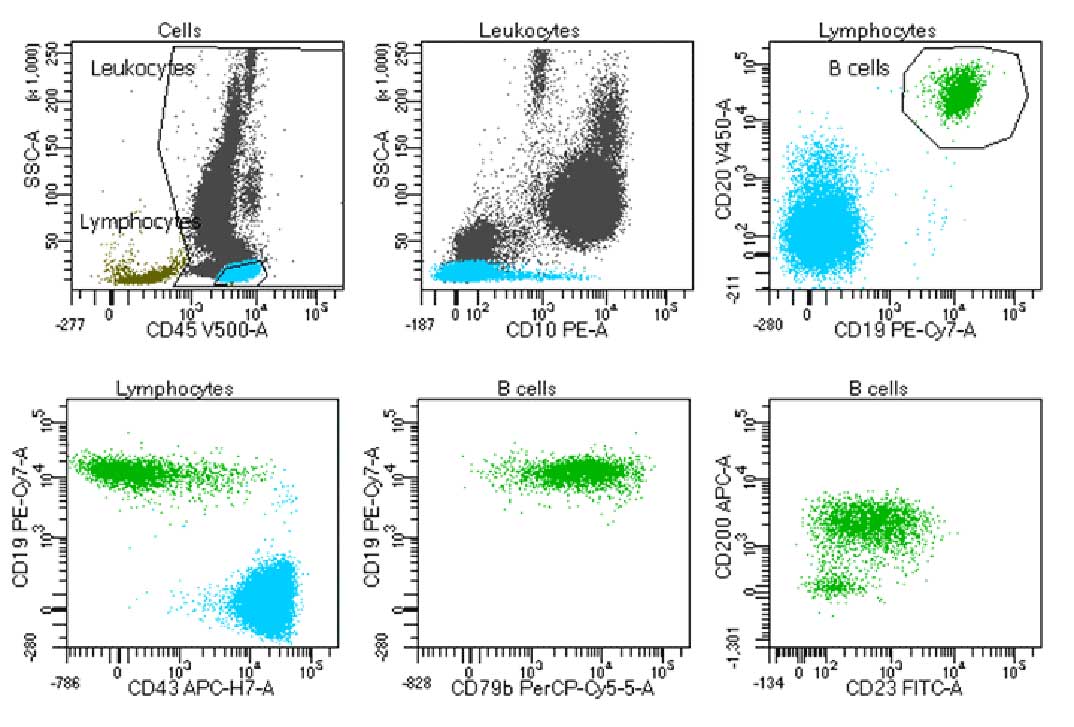
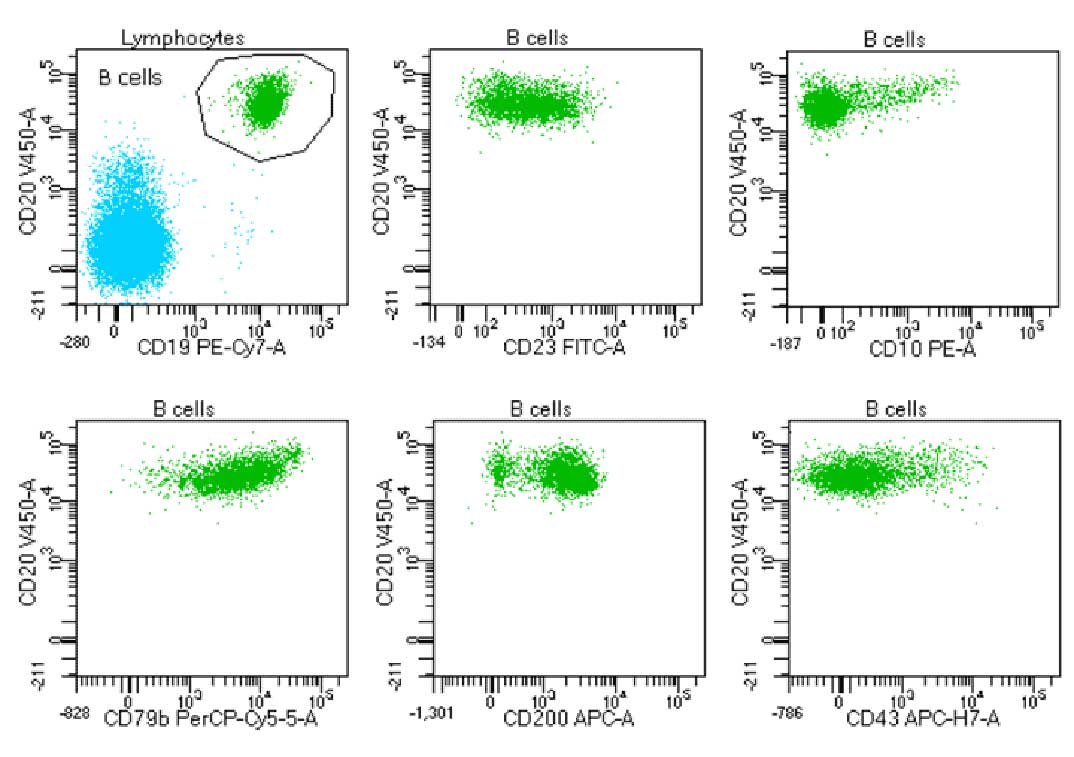
The BD OneFlow™ B-CLPD T1 reagent composition
| Antibody | Fluorochrome | Clone | Target Populations |
|---|---|---|---|
| CD23 | FITC | EBVCS-5 | Contributes to the classification of CLL or other B-cell chronic lymphoproliferative diseases |
CD10 | PE | MEM-78 | Contributes to the classification of CLL or other B-cell chronic lymphoproliferative diseases |
| CD79b | PerCP-Cy 5.5 | SN8 | Contributes to the classification of CLL or other B-cell chronic lymphoproliferative diseases |
| CD19 | PE-CY 7 | SJ25-C1 | Backbone marker. In common with BD OneFlow™ LST |
| CD200 | APC | MRC 0X-104 | Contributes to the classification of CLL or other B-cell chronic lymphoproliferative diseases |
| CD43 | APC-H7 | 1G10 | Contributes to the classification of CLL or other B-cell chronic lymphoproliferative diseases |
| CD20 | BD Horizon™ V450 | L27 | Backbone marker. In common with BD OneFlow™ LST |
| CD45 | BD Horizon™ V500-C | 2D1 | Backbone marker. In common with BD OneFlow™ LST |
The EuroFlow antibody panel article1* has a full description of the utility of the antibodies chosen for the BD OneFlow™ B-CLPD T1.
APPLICATIONS
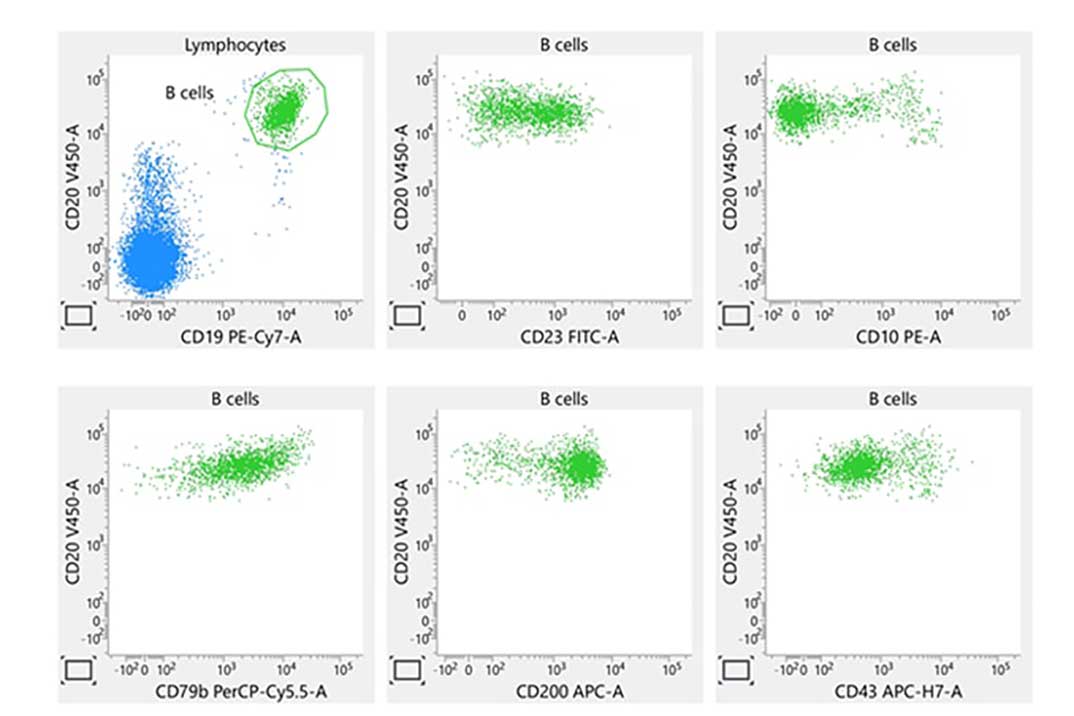
Laboratory report of a normal bone marrow specimen stained with the 8-color 8-antibody BD OneFlow™ B-CLPD T1 single-test reagent acquired on the BD FACSLyric™ Flow Cytometer with BD FACSuite™ Clinical Application v1.4.
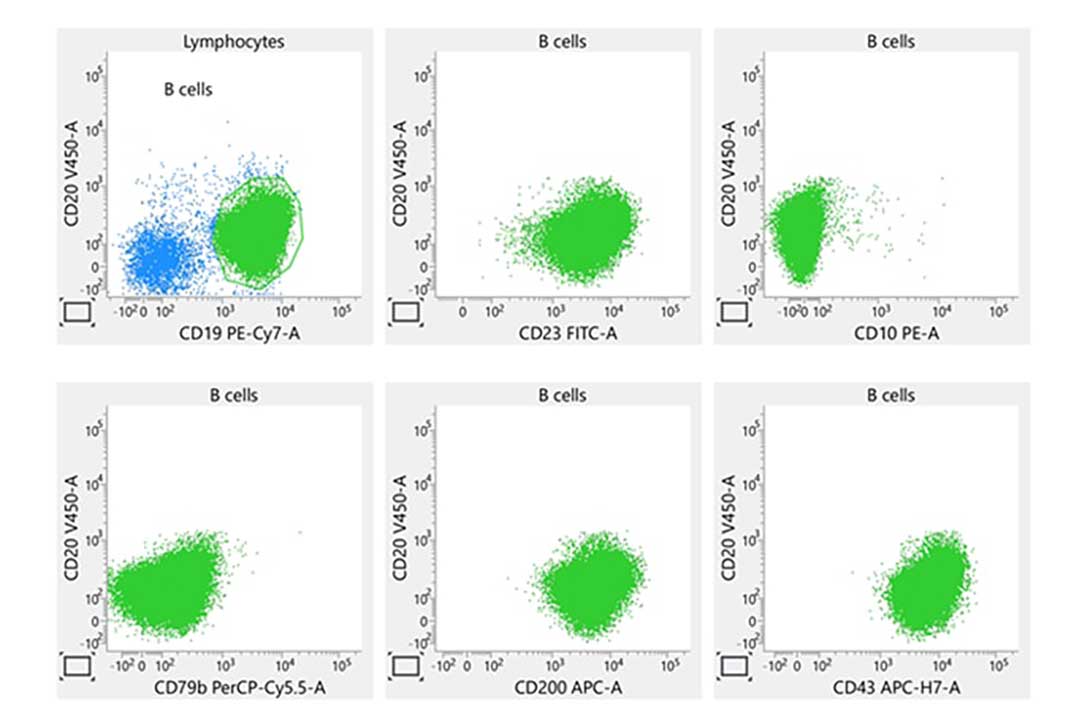
Laboratory report of an abnormal bone marrow specimen stained with the 8-color 8-antibody BD OneFlow™ B-CLPD T1 single-test reagent acquired on the BD FACSLyric™ Flow Cytometer with BD FACSuite™ Clinical Application v1.4.
Periodic and daily setup

-
Instructions for Use
-
Application Guides - For BD FACSLyric™ Flow Cytometer Users
-
Applications Guides - For BD FACSCanto™ II Flow Cytometer Users
-
Brochure
-
Tools
-
Application Note
-
Instructions for Use
-
Application Guides - For BD FACSLyric™ Flow Cytometer Users
-
Application Guides - For BD FACSCanto™ II Flow Cytometer Users
Standardization:
Reproducibility:
References
- van Dongen JJM, Lhermitte L, Böttcher S, et al. on behalf of the EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9): 1908-1975. doi: 10.1038/leu.2012.120
- Moloney E, Watson H, Barge D, et al. Efficiency and health economic evaluations of BD OneFlow™ Flow Cytometry Reagents for diagnosing chronic lymphoid leukemia. Cytometry B Clin Cytom. 2019;96(6):514-520. doi: 10.1002/cyto.b.21779
- Kalina T, Flores-Montero J, van der Velden VHJ, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986-2010. doi: 10.1038/leu.2012.122
The BD FACSLyric™ and BD FACSCanto™ II Flow Cytometers are Class 1 Laser Products.
BD OneFlow™ Reagents, BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ Applications, BD FACSCanto™ II Flow Cytometer, BD® FC Beads (8-Color, 7-Color, 5-Color and 2-Color Kits), BD OneFlow™ Setup Beads, BD FACSDiva™ CS&T IVD Beads and BD® CS&T Beads are CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC.
The BD OneFlow™ Solution is intended for professional use only.
The EuroFlow trademark is the property of the EuroFlow Consortium and cannot be reproduced or published without prior written permission from the EuroFlow coordinator (www.euroflow.org).
Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
*This publication refers to EuroFlow and is relevant to the BD OneFlow™ Solution as the BD OneFlow™ Solution demonstrated equivalent results to EuroFlow through a clinical trial.
23-23738-00