-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
-
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- Japan (Japanese)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?



.png)

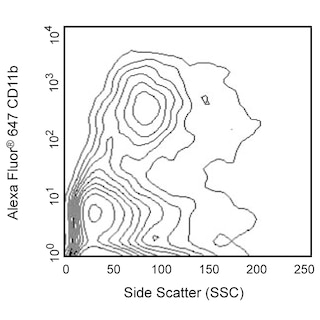
Flow cytometric analysis of CXCL9 (MIG) expression in Stimulated mouse peritoneal exudate cells (PEC). Thioglycolate-elicited BALB/c mouse PEC were cultured in complete tissue culture medium with Recombinant Mouse IFN-γ protein (Cat. No. 554587, 10 ng/ml) for 2 hours followed by the addition of lipopolysaccharide (LPS; Sigma, 1 μg/ml). BD GolgiPlug™ Protein Transport Inhibitor (Cat. No. 555029) was then added to both cultures that were further cultured overnight. The cells were harvested, washed, fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655), and then permeabilized with BD Perm/Wash™ Buffer (Cat. No. 554723). The cells were then stained in BD Perm/Wash™ Buffer with Alexa Fluor® 647 Rat Anti-Mouse CD11b (Cat. No. 557686) and either PE Armenian Hamster IgG1, κ Isotype Control (Cat. No. 553972; Left Plot) or PE Armenian Hamster Anti-Mouse CXCL9 (MIG) antibody (Cat. No. 567163; Left Plot) at 0.25 μg/test. The two-color flow cytometric contour plots showing CXCL9 (MIG) expression (or Ig Isotype control staining) versus CD11b were derived from gated events with the forward and side light-scatter characteristics of intact cells. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.

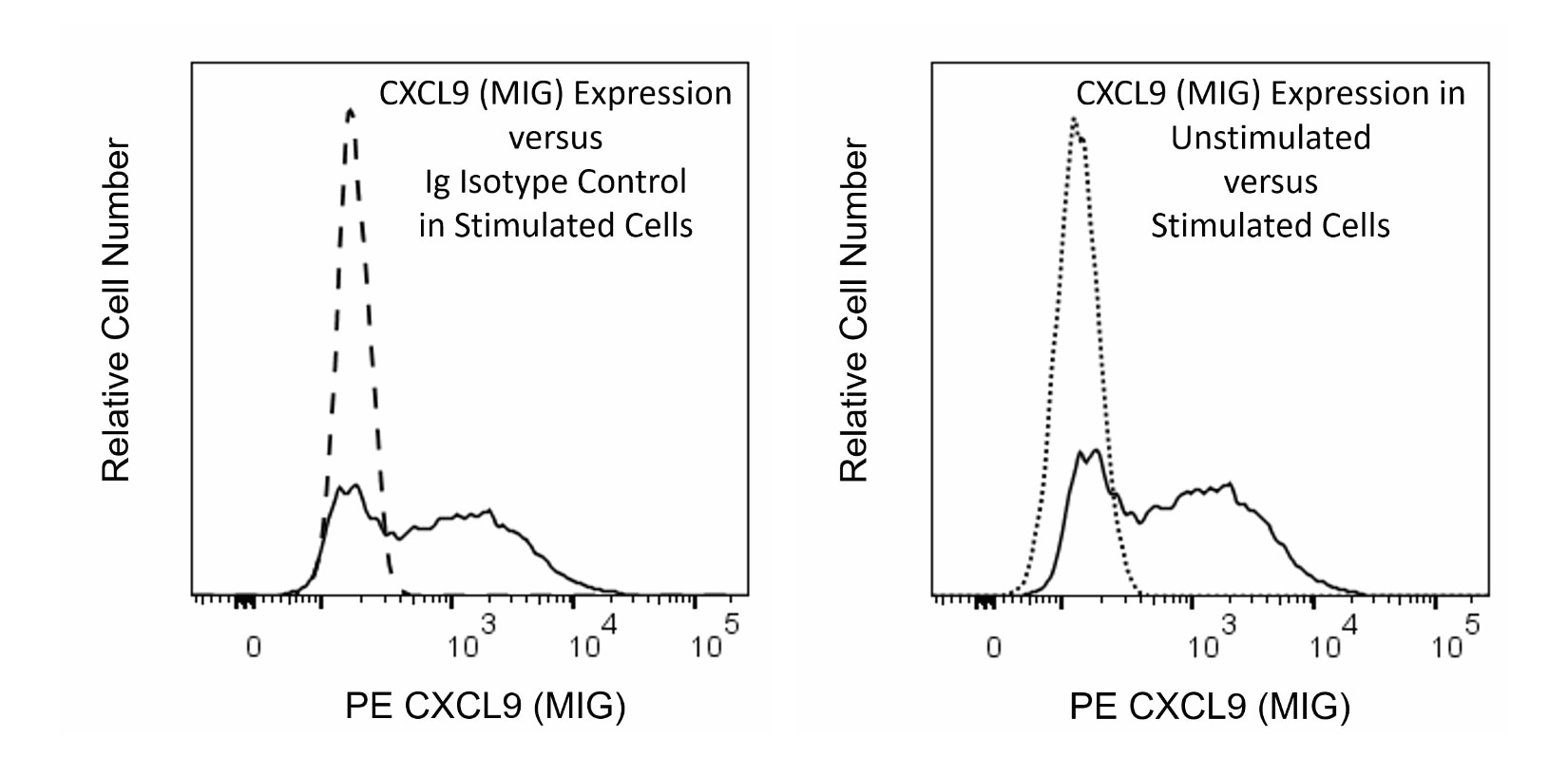
Flow cytometric analysis of CXCL9 (MIG) expression in Unstimulated and Stimulated RAW264.7 cells. Cells from the mouse RAW 264.7 (Macrophage; ATCC TIB-71) cell line were cultured in complete tissue culture medium without activators [Unstimulated Cells] or with Recombinant Mouse IFN-γ protein (Cat. No. 554587, 10 ng/ml) for 2 hours followed by the addition of lipopolysaccharide (LPS; Sigma, 1 μg/ml) [Stimulated Cells]. BD GolgiPlug™ Protein Transport Inhibitor (Cat. No. 555029) was then added to both cultures that were further cultured overnight. The cells were harvested, washed, fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655), and then permeabilized with BD Perm/Wash™ Buffer (Cat. No. 554723). The cells were then stained in BD Perm/Wash™ Buffer with PE Armenian Hamster IgG1, κ Isotype Control (Cat. No. 553972; dashed line histogram) or PE Armenian Hamster Anti-CXCL9 (MIG) antibody (Cat. No. 567163; solid and dotted line histograms) at 0.25 μg/test. Left Plot: The fluorescence histogram showing CXCL9 (MIG) expression (solid line) or Ig Isotype control staining (dashed line) were derived from gated events with the light-scatter characteristics of intact cells. Right Plot: The histograms showing CXCL9 (MIG) expression in Unstimulated Cells (dotted line) versus Stimulated Cells (solid line) were overlaid for the sake of direct comparison as shown. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.
.png)

BD Pharmingen™ PE Hamster Anti-Mouse CXCL9 (MIG)

BD Pharmingen™ PE Hamster Anti-Mouse CXCL9 (MIG)
.png)
Regulatory Statusの凡例
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation and Storage
推奨アッセイ手順
BD™ CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cell and CompBead to ensure that BD Comp beads are appropriate for your specific cellular application.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Although hamster immunoglobulin isotypes have not been well defined, BD Biosciences Pharmingen has grouped Armenian and Syrian hamster IgG monoclonal antibodies according to their reactivity with a panel of mouse anti-hamster IgG mAbs. A table of the hamster IgG groups, Reactivity of Mouse Anti-Hamster Ig mAbs, may be viewed at http://www.bdbiosciences.com/documents/hamster_chart_11x17.pdf.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
関連製品




.png?imwidth=320)
The MIG 2F5.5 monoclonal antibody specifically recognizes MIG (Monokine induced by gamma interferon) which is also known as C-X-C motif chemokine 9 (CXCL9) or Small-inducible cytokine B9 (Scyb9). Mouse CXCL9 (MIG) is encoded by Cxcl9 and belongs to the C-X-C family of chemokines. CXCL9 (MIG) production is induced in a variety of cells including macrophages, hepatocytes, and endothelial cells in response to gamma interferon (IFN-γ). The seven-transmembrane G-protein coupled receptor termed CXCR3, also known as CD183, serves as the cell surface signaling receptor for CXCL9 (MIG). This chemotactic factor attracts activated and memory CD4+ and CD8+ T cells including Type-1 (Th1-like) CD4+ T cells as well as natural killer (NK) cells. CXCL9 (MIG) plays important roles in promoting protective immunity through the attraction of CXCR3+ T cells and NK cells but can also function in inflammation and autoimmune diseases.
Development References (4)
-
Asai A, Tsuda Y, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Pathogenic role of macrophages in intradermal infection of methicillin-resistant Staphylococcus aureus in thermally injured mice.. Infect Immun. 2010; 78(10):4311-9. (Clone-specific: Flow cytometry). View Reference
-
Krug A, Uppaluri R, Facchetti F, et al. IFN-producing cells respond to CXCR3 ligands in the presence of CXCL12 and secrete inflammatory chemokines upon activation. J Immunol. 2002; 169(11):6079-6083. (Immunogen: ELISA). View Reference
-
Marcus AJ, Ullman HL, Safier LB. Lipid composition of subcellular particles of human blood platelets.. J Lipid Res. 1969; 10(1):108-14. (Biology). View Reference
-
Sung JH, Zhang H, Moseman EA, et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes.. Cell. 2012; 150(6):1249-63. (Clone-specific: Flow cytometry, Immunofluorescence). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.