-
抗体試薬
- フローサイトメトリー用試薬
-
ウェスタンブロッティング抗体試薬
- イムノアッセイ試薬
-
シングルセル試薬
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
細胞機能評価のための試薬
-
顕微鏡・イメージング用試薬
-
細胞調製・分離試薬
-
- BD® AbSeq Assay | シングルセル試薬
- BD Rhapsody™ Accessory Kits | シングルセル試薬
- BD® Single-Cell Multiplexing Kit | シングルセル試薬
- BD Rhapsody™ Targeted mRNA Kits | シングルセル試薬
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit | シングルセル試薬
- BD Rhapsody™ TCR/BCR Profiling Assays (VDJ Assays) | シングルセル試薬
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- Japan (Japanese)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




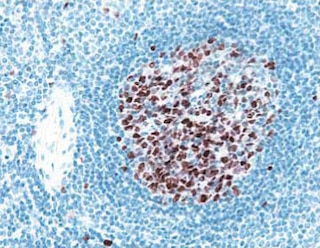
Analyses of NCAM-1 (CD56) expression by Immunohistochemistry and Flow Cytometry Left Panel: Immunohistochemical staining of human spleen for NCAM-1 (CD56). Following antigen retrieval with BD Retrievagen A Buffer (Cat. No. 550524), the formalin-fixed paraffin-embedded sections were stained with either Purified Mouse IgG1 κ Isotype Control (Cat. No. 550878; Left Image) or Purified Mouse Anti-Human NCAM-1 (CD56) antibody (Cat. No. 563237; Right Image). A three-step staining procedure that employs a Biotin Goat Anti-Mouse Immunoglobulin (Cat. No. 550337), Streptavidin-Horseradish Peroxidase (HRP) (Cat. No. 550946), and the DAB Substrate Kit (Cat. No. 550880) was used to develop the primary staining reagents. As shown in the Right Figure, the NCAM-1 (CD56)-specific antibody primarily surface stained some splenic lymphocytes. Original magnification: 20×. Middle Panel: Immunohistochemical staining of human small intestine was performed as described above for the spleen. Staining of some neurons of the Myenteric plexus was observed. Original magnification: 40×. Right Panel: Two-parameter flow cytometric analysis of NCAM-1 (CD56) expression by fixed and permeabilized human peripheral blood lymphocytes (PBL). Erythrocytes were lysed and leucocytes were fixed and permeabilized in a human whole blood sample using 1X BD Phosflow™ Lyse/Fix Buffer (Cat. No. 558049; 10 min, 37˚C) followed by BD Phosflow™ Perm Buffer III (Cat. No. 558050; 30 min, on ice). The leucocytes were then labeled with Purified Mouse Anti-Human NCAM-1 (CD56) antibody (Cat. No. 563237) followed by staining with PE Goat Anti-Mouse Ig (Cat. No.550589). The flow cytometric dot plot shows NCAM-1 (CD56) expression versus forward scattered light signals for gated events with the forward and side light-scatter characteristics of intact lymphocytes. Flow cytometry was performed on a BD FACSCanto™ II Flow Cytometry System.


BD Pharmingen™ Purified Mouse Anti-Human NCAM-1 (CD56)

Regulatory Statusの凡例
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation and Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Sodium azide is a reversible inhibitor of oxidative metabolism; therefore, antibody preparations containing this preservative agent must not be used in cell cultures nor injected into animals. Sodium azide may be removed by washing stained cells or plate-bound antibody or dialyzing soluble antibody in sodium azide-free buffer. Since endotoxin may also affect the results of functional studies, we recommend the NA/LE (No Azide/Low Endotoxin) antibody format, if available, for in vitro and in vivo use.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
関連製品


.png?imwidth=320)
The R19-760 monoclonal antibody specifically binds to NCAM-1 (Neural cell adhesion molecule 1) that is also known as CD56 and NKH1. NCAM-1 is a heavily glycosylated transmembrane protein and a member of the Ig supergene family. NCAM-1 represents an isoform of the neural cell adhesion molecule (NCAM). In the hematopoietic system, it is present on approximately 10% to 25% of peripheral blood lymphocytes. It is expressed on natural killer (NK) cells and a subset of T cells, NKT cells. It is not expressed on myeloid cells, erythrocytes or B cells. This molecule appears to function as an adhesion molecule and can serve as a panspecific NK-cell marker. The R19-760 antibody recognizes an epitope in the NCAM-1 (CD56) extracellular domain that resists destruction due to cellular fixation with BD Phosflow™ Lyse/Fix Buffer and permeabilization with BD Phosflow™ Perm Buffer III.
Development References (14)
-
Bennett IM, Zatsepina O, Zamai L, Azzoni L, Mikheeva T, Perussia B. Definition of a natural killer NKR-P1A+/CD56-/CD16- functionally immature human NK cell subset that differentiates in vitro in the presence of interleukin 12. J Exp Med. 1996; 184(5):1845-1856. (Biology). View Reference
-
Campbell JJ, Qin S, Unutmaz D, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001; 166(11):6477-6482. (Biology). View Reference
-
Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer–cell subsets. Trends Immunol. 2001; 22(11):633-640. (Biology). View Reference
-
Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987; 236(4803):799-806. (Biology). View Reference
-
Edelman GM. Cell adhesion molecules in the regulation of animal form and tissue pattern. Annu Rev Cell Biol. 1986; 2:81-116. (Biology). View Reference
-
Galandrini R, Tassi I, Mattia G, et al. SH2-containing inositol phosphatase (SHIP-1) transiently translocates to raft domains and modulates CD16-mediated cytotoxicity in human NK cells. Blood. 2001; 100(13):4581-4589. (Biology). View Reference
-
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002; 195(3):327-333. (Biology). View Reference
-
Lanier LL, Chang C, Azuma M, Ruitenberg JJ, Hemperly JJ, Phillips JH. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56). J Immunol. 1991; 146(12):4421-4426. (Biology). View Reference
-
Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986; 136(12):4480-4486. (Biology). View Reference
-
Lanier LL, Testi R, Bindl J, Phillips JH. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989; 169(6):2233-2238. (Biology). View Reference
-
Nitta T, Yagita H, Sato K, Okumura K. Involvement of CD56 (NKH-1/Leu-19 antigen) as an adhesion molecule in natural killer–target cell interaction. J Exp Med. 1989; 170(5):1757-1761. (Biology). View Reference
-
Phillips JH, Lanier LL. Dissection of the lymphokine-activated killer phenomenon: relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986; 164(3):814-825. (Biology). View Reference
-
Ritz J, Trinchieri G, Lanier LL. NK-cell Antigens: Section Report. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:1367-1372.
-
Schubert W, Zimmermann K, Cramer M, Starzinski-Powitz A. Lymphocyte antigen Leu-19 as a molecular marker of regeneration in human skeletal muscle. Proc Natl Acad Sci U S A. 1989; 86(1):307-311. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.