-
Reagents
- Flow Cytometry Reagents
-
蛋白质印迹试剂
- 免疫分析 试剂
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ 附件试剂盒
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- BD® OMICS-One Protein Panels
-
Functional Assays
-
显微成像试剂
-
Cell Preparation and Separation Reagents
-
- BD® AbSeq Assay
- BD Rhapsody™ 附件试剂盒
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- BD® OMICS-One Protein Panels
- China (Chinese)
-
改变地点/语言
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location or be switched to your location?
BD Pharmingen™ Purified Mouse anti-Human TRA-1-85 Antigen
克隆 TRA-1-85 (RUO)
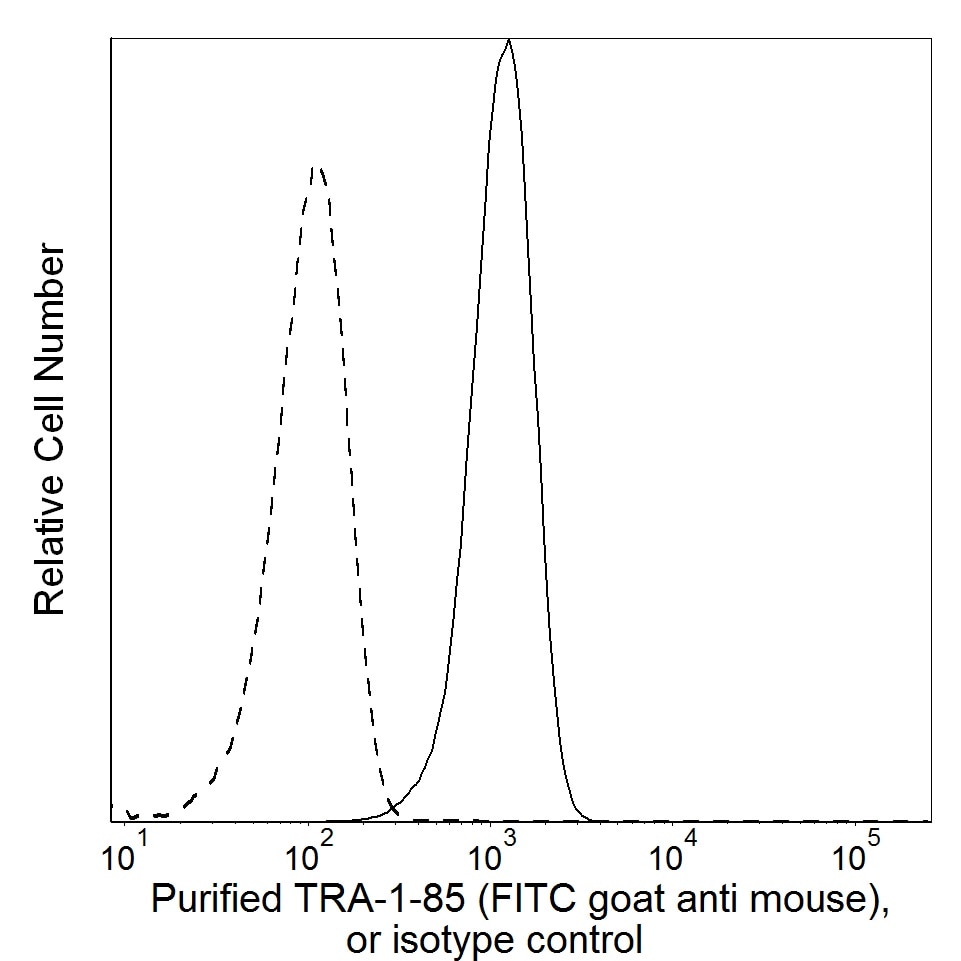
Flow cytometric analysis of TRA-1-85 expression on human cervical carcinoma (HeLa cell line). HeLa cells (ATCC® CCL 2™) were stained with Purified Mouse anti-Human TRA-1-85 Antigen monoclonal antibody (solid line histogram) or with Purified Mouse IgG1, κ Isotype Control (Cat. No. 554121; dashed line histogram). The second-step reagent was FITC goat anti-Mouse Ig (Cat. No. 554001). The fluorescence histograms were derived from events with the forward and side light-scatter characteristics of the HeLa cell line. Flow cytometry was performed using a BD LSRFortessa™ Flow Cytometer System.
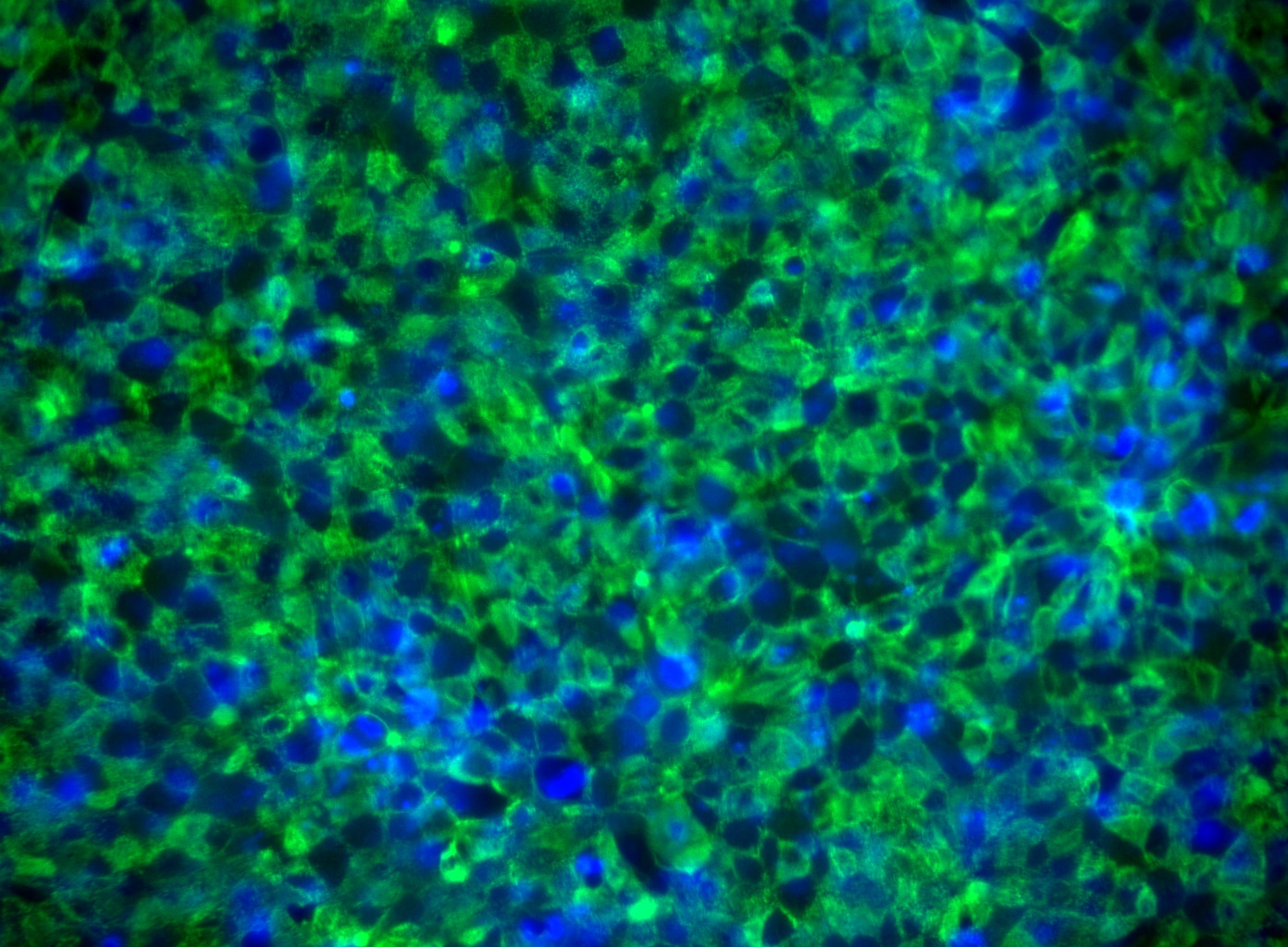
Immunoflourescent staining of Tra-1-85 on human embryonic stem (ES) cells. H9 human ES cells (WiCell, Madison, WI) passage 47 grown in mTESR™1 media (StemCell Technologies) on BD Matrigel™ hESC-qualified Matrix (Cat. No. 354277) were fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655). The cells were stained with Purified Mouse anti-Human TRA-1-85 Antigen monoclonal antibody (pseudo-colored green) at 1.2 μg/mL. The second-step reagent was Alexa Fluor® 488 goat anti-mouse Ig (Life Technologies), and cell nuclei were stained with Hoechst (pseudo-colored blue). The images were captured on a BD Pathway™ 435 Cell Analyzer and merged using BD Attovision™ software.



Flow cytometric analysis of TRA-1-85 expression on human cervical carcinoma (HeLa cell line). HeLa cells (ATCC® CCL 2™) were stained with Purified Mouse anti-Human TRA-1-85 Antigen monoclonal antibody (solid line histogram) or with Purified Mouse IgG1, κ Isotype Control (Cat. No. 554121; dashed line histogram). The second-step reagent was FITC goat anti-Mouse Ig (Cat. No. 554001). The fluorescence histograms were derived from events with the forward and side light-scatter characteristics of the HeLa cell line. Flow cytometry was performed using a BD LSRFortessa™ Flow Cytometer System.
Immunoflourescent staining of Tra-1-85 on human embryonic stem (ES) cells. H9 human ES cells (WiCell, Madison, WI) passage 47 grown in mTESR™1 media (StemCell Technologies) on BD Matrigel™ hESC-qualified Matrix (Cat. No. 354277) were fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655). The cells were stained with Purified Mouse anti-Human TRA-1-85 Antigen monoclonal antibody (pseudo-colored green) at 1.2 μg/mL. The second-step reagent was Alexa Fluor® 488 goat anti-mouse Ig (Life Technologies), and cell nuclei were stained with Hoechst (pseudo-colored blue). The images were captured on a BD Pathway™ 435 Cell Analyzer and merged using BD Attovision™ software.

Flow cytometric analysis of TRA-1-85 expression on human cervical carcinoma (HeLa cell line). HeLa cells (ATCC® CCL 2™) were stained with Purified Mouse anti-Human TRA-1-85 Antigen monoclonal antibody (solid line histogram) or with Purified Mouse IgG1, κ Isotype Control (Cat. No. 554121; dashed line histogram). The second-step reagent was FITC goat anti-Mouse Ig (Cat. No. 554001). The fluorescence histograms were derived from events with the forward and side light-scatter characteristics of the HeLa cell line. Flow cytometry was performed using a BD LSRFortessa™ Flow Cytometer System.

Immunoflourescent staining of Tra-1-85 on human embryonic stem (ES) cells. H9 human ES cells (WiCell, Madison, WI) passage 47 grown in mTESR™1 media (StemCell Technologies) on BD Matrigel™ hESC-qualified Matrix (Cat. No. 354277) were fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655). The cells were stained with Purified Mouse anti-Human TRA-1-85 Antigen monoclonal antibody (pseudo-colored green) at 1.2 μg/mL. The second-step reagent was Alexa Fluor® 488 goat anti-mouse Ig (Life Technologies), and cell nuclei were stained with Hoechst (pseudo-colored blue). The images were captured on a BD Pathway™ 435 Cell Analyzer and merged using BD Attovision™ software.




监管状态图例
未经BD明确书面授权,严禁使用未经许可的任何商品。
准备和存储
商品通知
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- mTESR™1 is a trademark of StemCell Technologies.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- All other brands are trademarks of their respective owners.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Sodium azide is a reversible inhibitor of oxidative metabolism; therefore, antibody preparations containing this preservative agent must not be used in cell cultures nor injected into animals. Sodium azide may be removed by washing stained cells or plate-bound antibody or dialyzing soluble antibody in sodium azide-free buffer. Since endotoxin may also affect the results of functional studies, we recommend the NA/LE (No Azide/Low Endotoxin) antibody format, if available, for in vitro and in vivo use.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
配套商品



The TRA-1-85 monoclonal antibody (mAb) specifically binds to the high frequency blood group antigen OKa. The OKa blood group antigen is a 35-69 kDa glycoprotein also known as CD147, EMMPRIN, M6, or Basigin. The OKa group is widely expressed on most human tissues including, but not limited to kidney, peripheral blood leukocytes, liver, pancreas, colon, skin, and brain. TRA-1-85 mAb is often used to discern human cells from non-human cells in multiple applications including animal transplantation studies. During development, the purified TRA-1-85 mAb was found to detect OKa blood group antigen by western blot analysis of cellular lysates, by immunofluorescent staining of fixed cells, and by flow cytometric analysis of live cells.
研发参考 (5)
-
Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009; 27(3):559-567. (Clone-specific: Flow cytometry). 查看参考
-
Draper JS, Pigott C, Thomson JA, Andrews PW. Surface antigens of human embryonic stem cells: changes upon differentiation in culture. J Anat. 2002; 200:249-258. (Clone-specific: Flow cytometry). 查看参考
-
Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006; 24(8):1914-1922. (Biology). 查看参考
-
Spring FA, Holmes CH, Simpson KL, et.al. The Oka blood group antigen is a marker for the M6 leukocyte activation antigen, the human homolog of OX-47 antigen, basigin and neurothelin, an immunoglobulin superfamily molecule that is widely expressed in human cells and tissues. Eur J Immunol. 1997; 27(4):891-897. (Clone-specific: Flow cytometry, Immunohistochemistry, Western blot). 查看参考
-
Williams BP, Daniels GL, Pym B, et al. Biochemical and genetic analysis of the OKa blood group antigen. Immunogenetics. 1988; 27:322-329. (Clone-specific: Western blot). 查看参考
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.