-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD Accuri™ C6 Plus Cell Analyzer
- BD FACSAria™ Cell Sorter Cell Sorter
- BD FACSCanto™ Cell Analyzer
- BD FACSDiscover™ A8 Cell Analyzer
- BD FACSDiscover™ S8 Cell Sorter
- BD FACSDuet™ Sample Preparation System
- BD FACSLyric™ Cell Analyzer
- BD FACSMelody™ Cell Sorter
- BD FACSymphony™ Cell Analyzer
- BD LSRFortessa™ Cell Analyzer
- Advanced Training
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Panel Design
Multicolor flow cytometry is a powerful technology that enables simultaneous analysis of multiple markers at the single-cell level. With the increase in number of detectable parameters, the design of a multicolor panel can be challenging and requires an understanding of several factors that can influence panel performance:
- Biology—Antigen density and co-expression
- Fluorochrome—Brightness and spillover
- Instrument—Configuration and set-up
- Explore pre-designed panels at the Interactive Cell Map
Step 1
Define your experimental hypothesis
Defining your experimental hypothesis is the first step in panel design. Start with identifying:
- The biological information you are trying to achieve
- The population(s) of cells you wish to interrogate
- Whether targets are found on the cell surface or intracellularly

Watch the flow cytometry panel design step 1 video to begin your panel design journey.
Step 2
Marker selection
During the second step of the panel design process, you will need to identify which and how many markers you need to identify the population of interest.
Pay attention to:
- Marker expression levels
- Primary antigen: Expressed at high density, often defining lineages
- Secondary antigen: Often expressed over a continuum
- Tertiary antigen: Critical markers expressed at low density
- Marker coexpression, especially of dim markers
- The gating strategy needed to identify the population(s) of cells you wish to interrogate
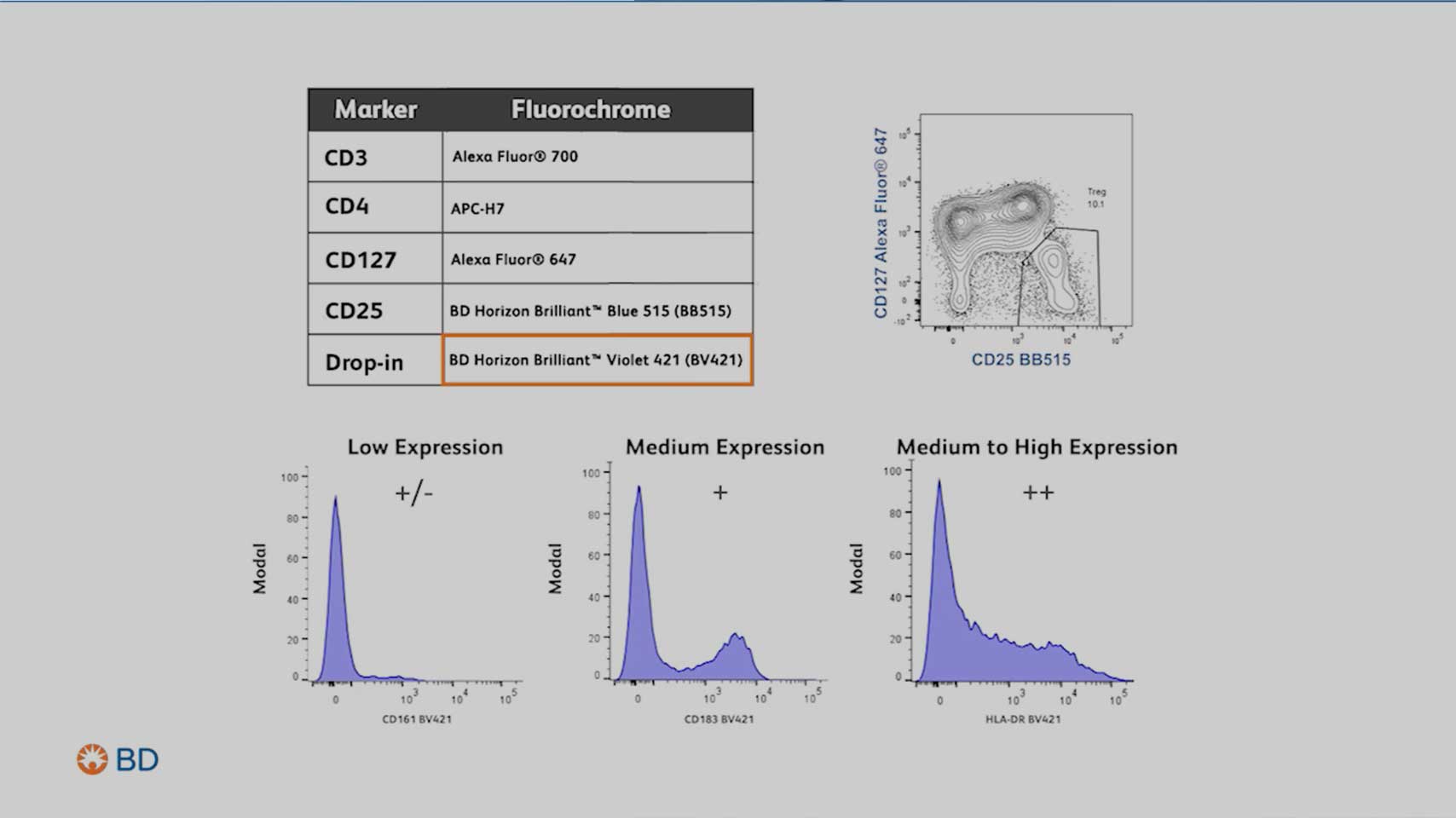
Watch the flow cytometry panel design step 2 video to understand the intricacies in identifying critical populations and selecting your markers for analysis.
Step 3
Know your flow cytometer
Knowing your instrument is essential. Understanding your instrument's configuration will let you know how many markers and which fluorochromes your instrument can detect.
Elements to consider include:
- Laser wavelength for excitation
- Number of detectors for each laser
- Filters available to detect the fluorochromes

Watch the flow cytometry panel design step 3 video to understand and maximize the capabilities of your flow cytometer for an optimal panel design.
Step 4
Fluorochrome assignment
Carefully select fluorochromes to resolve markers at all expression levels and minimize spectral overlap. Consider using tools like a fluorochrome resolution ranking and a spectrum viewer to help assess:
- Fluorochrome resolution
- Cross laser excitation
- Fluorochrome spillover
Remember to pair bright fluorochromes with low expressing antigens and dim fluorochromes with high expressors. Keep in mind that spread only impacts the resolution of coexpressed markers.

Watch the flow cytometry panel design step 4 video to learn strategies for matching fluorochromes and antigens for optimal panel design.
Step 5
Review panel
Review your panel design and begin ordering your reagents. Remember to titrate your mass size reagents and optimize your staining protocol. Include proper controls for compensation, FMO and biological controls to help ensure optimal panel performance.
To find more panel design tips and some fun facts, download the “Flow cytometry panel design journey” infographic.

Multicolor flow cytometry panel design videos
Add A Solid Foundation for Simpler and Smarter Panel Design
A comprehensive overview of panel design fundamentals and different approaches to simplify panel design.
Flow Cytometry Instrument Characterization and Set Up for Optimal Panel Design
This video explains how to leverage flow cytometry configurations to help maximize resolution of populations of interest.

Avoiding Resolution Loss When Using Fix and Permeabilization Buffers for Intracellular Staining
In this video you will learn helpful strategies to help prevent loss of resolution due to intracellular fixation and permeabilization buffers.

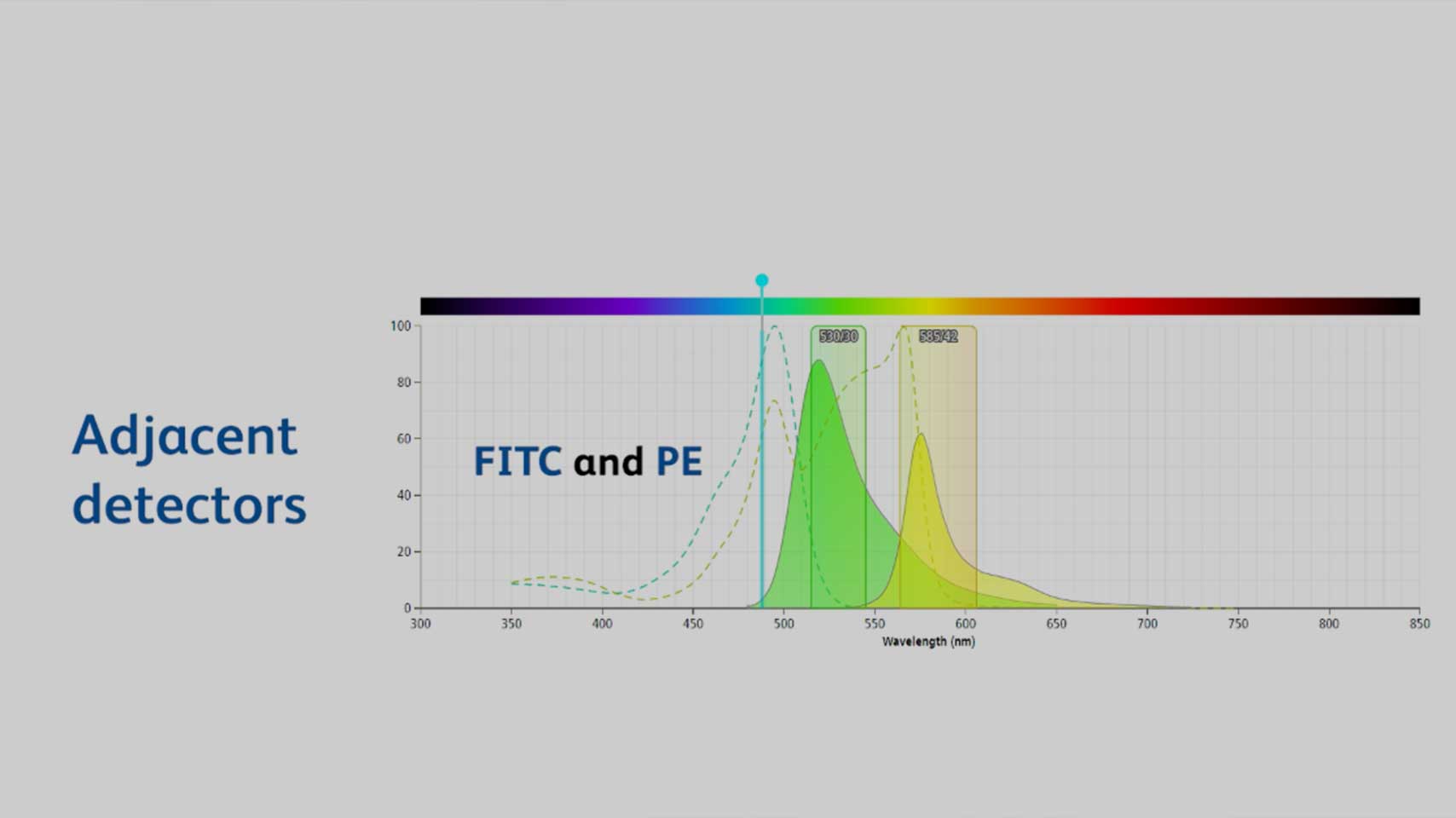
Understanding and Managing Fluorescence Spillover
In this video you will learn how to manage fluorescence spillover to get the most out of your flow cytometry experiments.
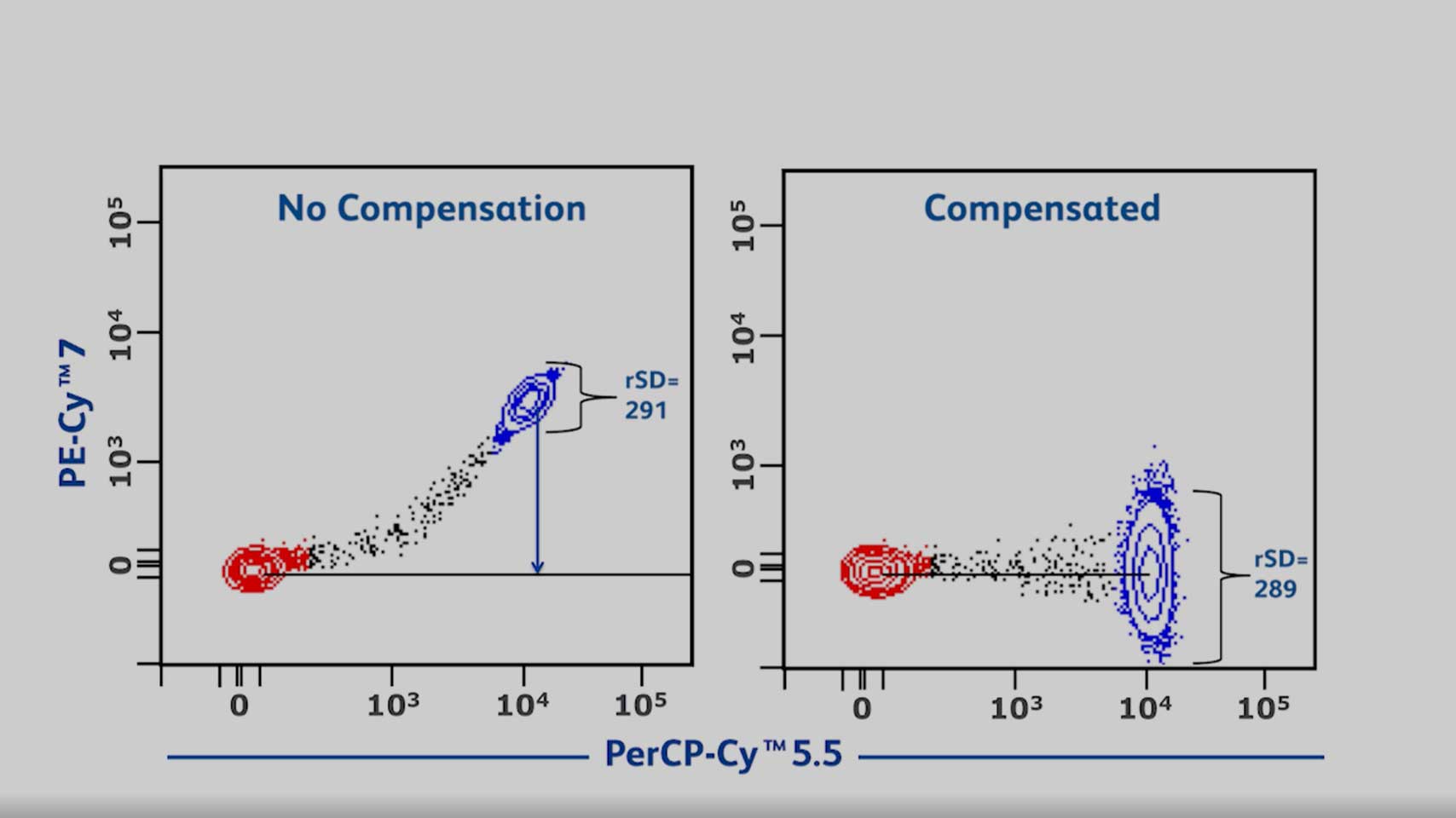
Relationship Between Fluorescence Spillover and Spread
This video will help you understand the relationship between florescence spillover and spread to help you implement the right strategies to resolve your populations of interest.
Minimizing the Impact of Spread in Flow Cytometry Experiments
This video provides strategies to help minimize the impact of spread in flow cytometry experiments to help improve resolution.

Panel Design Workflow for Optimal Fluorochrome-Antigen Matching
This video provides simple strategies for fluorochrome-antigen matching to help prevent the introduction of spread and subsequent resolution loss.

BD Horizon™ Tour Series
This webinar explores concepts, best practices and available tools to help you efficiently navigate multicolor flow cytometry.
Speaker
Andrew D. Bantly
Technical Applications Specialist
Discover a new way of looking at fluorochromes and markers that can maximize the number of parameters achievable on any instrument.
Speaker
Bob Balderas, MBA
VP Biological Sciences & VP Market Development
BD Life Sciences–Biosciences
This webinar offers a comprehensive look at best practices for managing instrument set up to help you maximize your instrument’s parameters while minimizing spillover and resolution loss.
Speaker
Alan Stall
Principle Scientist
BD Life Sciences–Biosciences
This webinar explores the importance of understanding antigen density (the number of cell surface receptors on a cell) in relation to antibody/fluorochrome combinations for optimal panel design.
Speaker
Bob Balderas, MBA
VP Biological Sciences & VP Market Development
BD Life Sciences–Biosciences
This webinar explores the journey to finding what nature is hiding using flow cytometry by understanding the biology of your cells and the relative brightness of fluorochromes.
Speaker
Bob Balderas, MBA
VP Biological Sciences & VP Market Development
BD Life Sciences–Biosciences
BD Harmony Series
This webinar offers viewers practical strategies for designing optimized multicolor flow cytometry panels by addressing frequently asked questions.
Speakers
Bob Balderas, MBA
VP Biological Sciences & VP Market Development
BD Life Sciences–Biosciences
Mirko Corselli, PhD
Global Scientific Content Manager
BD Life Sciences–Biosciences
Rui Gardner, PhD
Head of Flow Cytometry Core Facility
Memorial Sloan Kettering Cancer Center
Yolanda Mahnke, PhD
Founder
FlowKnowHow LLC
This webinar is part of the Harmony Series and offers viewers panel design best practices, with emphasis on spillover and identification of optimal fluorochrome combinations to simplify panel design.
Speaker
Mirko Corselli, PhD
Global Scientific Content Manager
Research Solutions, BD Life Sciences–Biosciences
This webinar explores strategies to help customers create a solid chromatic foundation for multicolor panel design
Speakers
Bob Balderas, MBA
VP Biological Sciences & VP Market Development
BD Life Sciences–Biosciences
Other
This webinar explores strategies for minimizing flow cytometry panel design pain points as well as panel optimization
Speaker
Alan Stall
BD Fellow, Principal Scientist, BD Life Sciences–Biosciences
This webinar offers comprehensive strategies for adding efficiency to the flow cytometry panel design process
Speaker
Anis Larbi, PhD
Head of A*STAR Flow Cytometry Facility, Immuno-monitoring Platform and Principal Investigator, Biology of Aging Program
Singapore Immunology Network (SIgN)
-
Brochures
-
Posters
-
Tools
-
An Introduction to Compensation for Multicolor Assays on Digital Flow Cytometers
-
Fluorochrome Performance Chart
-
BD Biosciences Fluorochrome/Laser Reference Chart
-
Relative Fluorochrome Resolution Chart
-
Human and Mouse CD Marker Handbook
-
Mouse Leukocyte Alloantigens Chart
-
Flow Cytometry Workflow Journey
Mahnke YD, Roederer M. Optimizing a multicolor immunophenotyping assay. Clin Lab Med. 2007;27(3): 469–485, v. doi: 10.1016/j.cll.2007.05.002
Mair F, Tyznik A. High-dimensional immunophenotyping with fluorescence-based cytometry: a practical guidebook. Methods Mol Biol. 2019;2032:1-29. doi: 10.1007/978-1-4939-9650-6_1
Nguyen R, Perfetto S, Mahnke YD, Chattopadhyay P, Roederer M. Quantifying spillover spreading for comparing instrument performance and aiding in multicolor panel design. Cytometry A. 2013;83(3):306-315. doi: 10.1002/cyto.a.22251
Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45(3):194-205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c