-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® OMICS-One Protein Panels
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD Accuri™ C6 Plus Cell Analyzer
- BD FACSAria™ Cell Sorter Cell Sorter
- BD FACSCanto™ Cell Analyzer
- BD FACSDiscover™ A8 Cell Analyzer
- BD FACSDiscover™ S8 Cell Sorter
- BD FACSDuet™ Sample Preparation System
- BD FACSLyric™ Cell Analyzer
- BD FACSMelody™ Cell Sorter
- BD FACSymphony™ Cell Analyzer
- BD LSRFortessa™ Cell Analyzer
- Advanced Training
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® OMICS-One Protein Panels
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
-
- BD Accuri™ C6 Plus Cell Analyzer
- BD FACSAria™ Cell Sorter Cell Sorter
- BD FACSCanto™ Cell Analyzer
- BD FACSDiscover™ A8 Cell Analyzer
- BD FACSDiscover™ S8 Cell Sorter
- BD FACSDuet™ Sample Preparation System
- BD FACSLyric™ Cell Analyzer
- BD FACSMelody™ Cell Sorter
- BD FACSymphony™ Cell Analyzer
- BD LSRFortessa™ Cell Analyzer
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD™ Cytometric Bead Array (CBA) Human TGF-β1 Single Plex Flex Set
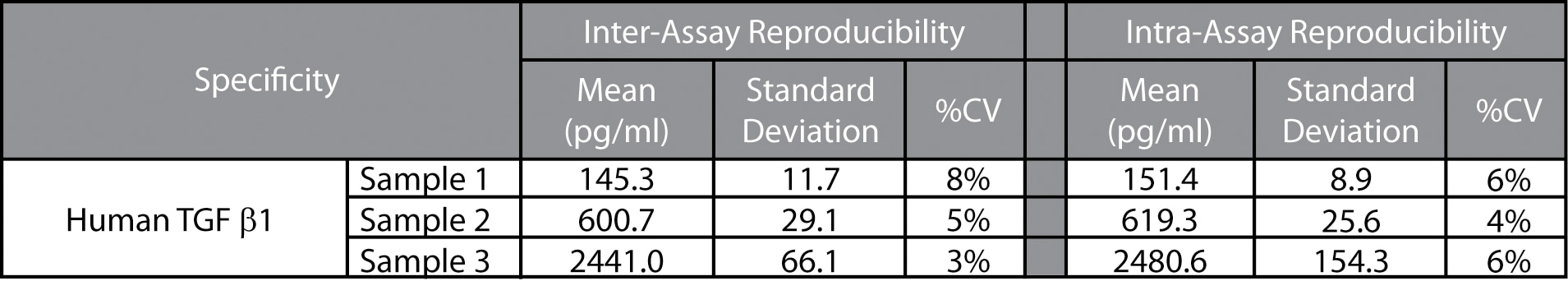
Reproducibility: The intra-assay and inter-assay reproducibility were determined for the BD CBA Human TGF-β1 Flex Set by evaluating ten replicates of three different sample levels (intra-assay) and three replicates of three different sample levels from four separate experiments (inter-assay).
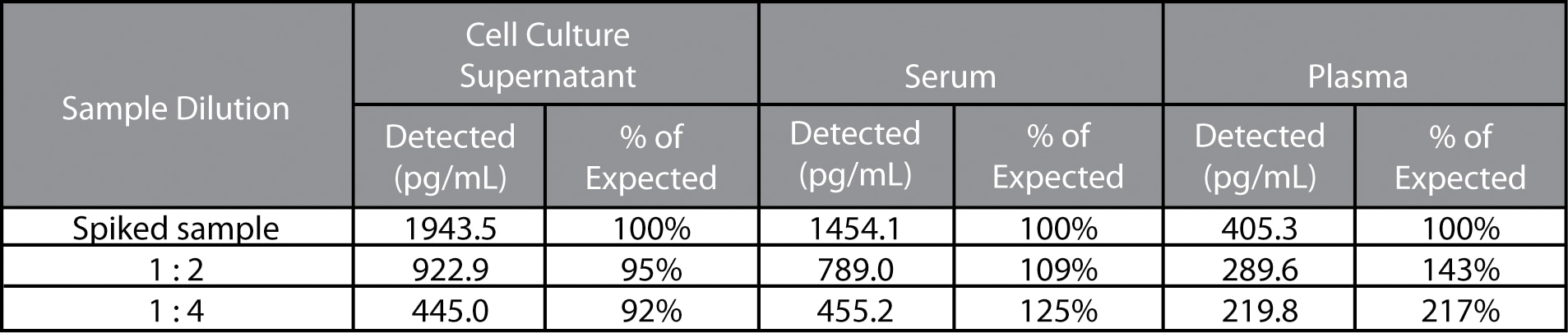
Linearity: Acid treated and diluted cell culture supernatant, serum or EDTA-treated plasma were spiked with protein and serially diluted. The diluted samples were assayed and the results were compared with the original spiked sample. The poor linearity suggests that this assay may not be optimal for plasma samples.
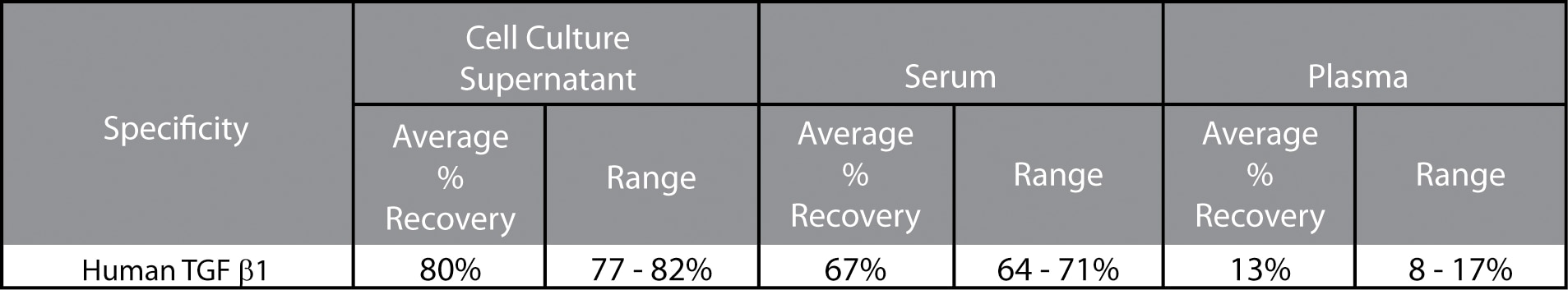
Recovery: Acid treated and diluted cell culture supernatant, serum, or EDTA-treated plasma were spiked with three different levels of protein. The spiked samples were assayed and the results were compared with expected values. Serum is a pool of 800 - 1000 donors and the plasma was pooled from at least 20 donors. The low recovery suggests that this assay may not be optimal for plasma samples.

Linearity: Acid treated and diluted cell culture supernatant, serum or EDTA-treated plasma were spiked with protein and serially diluted. The diluted samples were assayed and the results were compared with the original spiked sample. The poor linearity suggests that this assay may not be optimal for plasma samples.





Reproducibility: The intra-assay and inter-assay reproducibility were determined for the BD CBA Human TGF-β1 Flex Set by evaluating ten replicates of three different sample levels (intra-assay) and three replicates of three different sample levels from four separate experiments (inter-assay).
Linearity: Acid treated and diluted cell culture supernatant, serum or EDTA-treated plasma were spiked with protein and serially diluted. The diluted samples were assayed and the results were compared with the original spiked sample. The poor linearity suggests that this assay may not be optimal for plasma samples.
Recovery: Acid treated and diluted cell culture supernatant, serum, or EDTA-treated plasma were spiked with three different levels of protein. The spiked samples were assayed and the results were compared with expected values. Serum is a pool of 800 - 1000 donors and the plasma was pooled from at least 20 donors. The low recovery suggests that this assay may not be optimal for plasma samples.
Linearity: Acid treated and diluted cell culture supernatant, serum or EDTA-treated plasma were spiked with protein and serially diluted. The diluted samples were assayed and the results were compared with the original spiked sample. The poor linearity suggests that this assay may not be optimal for plasma samples.

Figure 1. Example BD CBA Human Soluble Human TGF-β1 Flex Set standard curve. Data acquired on a BD FACSArray™ bioanalyzer and analyzed using the FCAP Array™ Software (Cat. No. 641488).

Reproducibility: The intra-assay and inter-assay reproducibility were determined for the BD CBA Human TGF-β1 Flex Set by evaluating ten replicates of three different sample levels (intra-assay) and three replicates of three different sample levels from four separate experiments (inter-assay).

Linearity: Acid treated and diluted cell culture supernatant, serum or EDTA-treated plasma were spiked with protein and serially diluted. The diluted samples were assayed and the results were compared with the original spiked sample. The poor linearity suggests that this assay may not be optimal for plasma samples.


Recovery: Acid treated and diluted cell culture supernatant, serum, or EDTA-treated plasma were spiked with three different levels of protein. The spiked samples were assayed and the results were compared with expected values. Serum is a pool of 800 - 1000 donors and the plasma was pooled from at least 20 donors. The low recovery suggests that this assay may not be optimal for plasma samples.

Linearity: Acid treated and diluted cell culture supernatant, serum or EDTA-treated plasma were spiked with protein and serially diluted. The diluted samples were assayed and the results were compared with the original spiked sample. The poor linearity suggests that this assay may not be optimal for plasma samples.

ImageTitle~BD™ Cytometric Bead Array (CBA) Human TGF-β1 Single Plex Flex Set

ImageTitle~BD™ Cytometric Bead Array (CBA) Human TGF-β1 Single Plex Flex Set

ImageTitle~BD™ Cytometric Bead Array (CBA) Human TGF-β1 Single Plex Flex Set


ImageTitle~BD™ Cytometric Bead Array (CBA) Human TGF-β1 Single Plex Flex Set

ImageTitle~BD™ Cytometric Bead Array (CBA) Human TGF-β1 Single Plex Flex Set
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The BD™ Human TGF-β1 Flex Set is a bead-based immunoassay capable of measuring soluble human TGF-β1 in serum and cell culture supernatant samples. Human reactivity was determined by testing samples with the BD CBA Human TGF-β1 Flex Set. The biology and function of TGF-β1 has been extensively reviewed in the literature.
The BD CBA Human TGF-β1 Flex Set is a single plex assay. The BD CBA HumanTGF-β1 Flex Set should not be used with the following:
- BD CBA Human, Mouse or Rat Soluble Protein Flex Sets
- BD CBA Cell Signaling Flex Sets
- BD CBA Human Soluble Receptor Protein Flex Sets
- BD CBA Human Immunoglobulin Flex Sets
- Non-BD Bead Based Assays
Note: Plasma samples not recommended with this assay.
Recommended Assay Procedures
TGF-β1 Activation Reagent Preparation
The following reagents are needed to activate the latent TGF-β1 to its immunoreactive form:
Reagents for cell culture supernatant samples
1. 1 N HCl (100mL) - To 91.67 mL deionized water, slowly add 8.33 mL of 12 N HCl. Mix well.
2. 1.2 N NaOH/0.5 M HEPES (100mL) - To 75 mL of deionized water, slowly add 12 mL of 10 N NaOH. Mix well. Add 11.9 g of HEPES. Mix well. Bring the final volume to 100 mL with deionized water.
Reagents for serum samples
1. 2.5 N Acetic acid/8 M Urea (100mL) - To 40 mL of deionized water, slowly add 48.1 g of Urea. Mix until dissolved. Slowly add 14.4 mL of glacial acetic acid. Mix well. Bring the final volume to 100 mL with deionized water.
2. 2.7 N NaOH/1 M HEPES (100mL) - To 56 mL deionized water, slowly add 27 mL of 10 N NaOH. Mix well. Add 23.8 g of HEPES. Mix well. Bring the final volume to 100 mL with deionized water.
TGF-β1 Sample Activation
To activate the latent TGF-β1 to its immunoreactive form, samples have to be acidified to pH 3.0 or lower for a period of time and neutralized back to pH 7.2 - 7.6 before testing. Use polypropylene tubes. Do not activate the Flex Set standard. Recommended dilutions are a guideline, optimization may be required.
Activation of cell culture supernatant samples
1. Add 0.1 mL of 1 N HCl to 0.5 mL of sample and mix well.
2. Incubate for 10 minutes at RT.
3. Neutralize by adding 0.1 mL of 1.2 N NaOH/0.5 M HEPES and mix well.
4. The sample is now ready for testing.
5. The calculated concentration of samples run in the assay should be multiplied by a dilution factor of 1.4.
Activation of serum samples
1. Add 0.1 mL of 2.5 N Acetic Acid/8 M Urea to 0.1 mL of sample and mix well.
2. Incubate for 10 minutes at RT.
3. Neutralize by adding 0.1 mL of 2.7 N NaOH/1 M HEPES and mix well.
4. Dilute serum samples 10-fold with assay diluent prior to performing the assay.
5. The calculated concentration of samples run in the assay should be multiplied by a dilution factor of 30.
Note: TGF-β1 is highly conserved among species and this assay cross-reacts with multiple species, including mouse, rat, bovine, and porcine. Therefore, conditioned medium containing mammalian serum should be expected (when acidified) to have a significant background level of TGF-β1, which may vary depending on the species of the serum, batch, and percentage of serum used. There are several ways to deal with this background level of TGF-β1:
1. Determine the background TGF-β1 level in the culture medium alone and subtract it from the sample values.
2. Use mammalian serum-free medium for cell culture.
3. Switch to serum-free medium for desired period of time before harvesting samples.
Preparation of BD CBA Human TGF-β1 Flex Set Standard
A standard curve will need to be prepared. The protocol below indicates how the standard should be diluted for use in a BD CBA Human TGF-β1 Flex Set. Please follow the instructions below as they differ from those provided in the Human Soluble Protein Master Buffer Kit Manual. Store lyophilized standard and other components at 4°C.
1. Open one vial of lyophilized standard.
2. Transfer the lyophilized standard sphere into a polypropylene tube. Label tube "Top Standard". Investigators are strongly advised to transfer the lyophilized standard to the polypropylene tube for reconstitution as other methods may result in increased variability.
4. Reconstitute the standard with 1.0 mL of Assay Diluent. Allow the reconstituted standard to equilibrate for at least 15 minutes before making dilutions. Mix reconstituted protein by pipette only. Do not vortex or mix vigorously. When reconstituted in 1.0 ml of Assay Diluent, the standard has a protein concentration of 10,000 pg/ml. Discard unused reconstituted standard, do not store or reuse.
5. Label 12 × 75 mm tubes and arrange them in the following order: 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128 and 1:256.
6. Pipette 500 μL of Assay Diluent to each of the remaining tubes.
7. Perform a serial dilution by transferring 500 μL from the Top Standard to the 1:2 dilution tube and mix thoroughly (Mix by pipette only, do not vortex). Continue making serial dilutions by transferring 500 μL from the 1:2 tube to the 1:4 tube and so on to the 1:256 tube. Prepare one tube containing Assay Diluent to serve as the 0 pg/mL negative control.
8. It is recommended that the first ten wells or tubes in the experiment be the standards. Standards should be run in order from least concentrated (0 pg/ml) to most concentrated (Top Standard).
Preparation of BD CBA Human TGF-β1 Flex Set Capture Beads
The Capture Beads provided in the BD CBA Human TGF-β1 Flex Set are at a 50× concentration and must be diluted to their optimal concentration in the Capture Bead Diluent buffer before adding to a given assay tube or assay well. The Capture Bead Diluent for Serum/Plasma provided in the Human Soluble Protein Master Buffer Kit is not used in this assay, even if testing serum samples.
1. Determine the number of tests in the experiment. It is recommended that the user prepare a few additional tests than they will use in the experiment to ensure that there is enough material prepared for the experiment.
2. Vortex the Capture Beads stock vial for at least 15 seconds to resuspend the beads thoroughly.
3. Determine the total volume of diluted beads needed for the experiment. Each tube/well requires 50 μL of the diluted beads. The total volume of diluted beads can be calculated by multiplying the number of tests (determined in step 1) by 50 μL.
4. Determine the volume needed for the Capture Beads. Beads are supplied so that 1.0 μL = 1 test. Therefore, the required volume (μL) of beads is equal to the number of tests.
5. Determine the volume of Capture Bead Diluent needed to dilute the beads. The volume of Capture Bead Diluent can be calculated by subtracting the volume of beads from the total volume of diluted beads needed to perform the assay.
6. Pipette the Capture Beads and Capture Bead Diluent into a tube labeled Diluted Capture Beads.
Preparation of BD CBA Human TGF-β1 Flex Set PE Detection Reagent
The PE Detection Reagent provided with the BD CBA Human TGF-β1 Flex Set is a 50× bulk (1 μl/test) and should be diluted to its optimal volume per test (50 μl/test) before adding the PE Detection Reagent to a given tube or assay well.
Note: Protect the PE Detection Reagent from exposure to direct light because they can become photobleached and will lose fluorescent intensity.
1. Determine the number of tests to be run in the experiment. It is recommended that the user prepare a few additional tests than they will use in the experiment to ensure that there is enough material prepared for the experiment.
2. Determine the total volume of diluted PE Detection Reagent needed for the experiment. Each tube/well requires 50 μL of the diluted PE Detection Reagent. The total volume of diluted PE can be calculated by multiplying the number of tests (determined above).
3. Determine the volume needed for the TGF-β1 PE Detection Reagent. The PE Detection Reagent is supplied so that 1.0 μL = 1 test. Therefore, the required volume (μL) of PE Detection Reagent is equal to the number of tests.
4. Determine the volume of Detection Reagent Diluent needed to dilute the PE Detection Reagents. The volume of Detection Reagent Diluent can be calculated by subtracting the volume of the TGF-β1 PE Detection Reagent tested from the total volume of diluted PE needed.
5. Pipette the Detection Reagent and Detection Reagent Diluent into a tube labeled TGF-β1 PE Detection Reagent. Store at 4°C, protected from light until ready to use.
BD CBA Human TGF-β1 Flex Set Assay Procedure
Following the preparation and dilution of the individual assay components transfer the Standards or samples, Capture Beads, and PE Detection Reagents to the appropriate assay wells or tubes for incubation and analysis. Please follow the instructions below as they may differ from those provided in the Human Soluble Protein Master Buffer Kit Manual. Investigators should note that in some cases, additional Wash Buffer may be needed, which may be purchased separately (Cat. No. 560105).
Note: Protect Capture Beads and PE Detection Reagents from direct exposure to light.
For Plates:
1. Prepare all reagents as described in previous sections before starting the experiment.
2. Pre-wet the plate by adding 100 μL of Wash Buffer to each well. To remove the excess volume, apply to vacuum manifold. Do not exceed 10" Hg of vacuum pressure. Aspirate until wells are drained (2 - 10 seconds).
3. Vortex the diluted Capture Beads for at least 5 seconds. Add 50 μL of the diluted Capture Beads to each assay well.
4. Add 50 μL of standard or samples to the assay wells.
5. Mix the plate for 5 minutes using a digital shaker at 500 RPM (do not exceed 600 RPM) and incubate plate for 2 hours at RT.
6. Apply the plate to the vacuum manifold and vacuum aspirate (do not exceed 10" Hg of vacuum pressure) until wells are drained (2 - 10 seconds).
7. Add 50 μL of the diluted PE Detection Reagent to each assay well.
8. Mix the plate for 5 minutes using a digital shaker at 500 RPM and incubate plate at RT for 2 hours.
9. Apply the plate to the vacuum manifold and vacuum aspirate (do not exceed 10" Hg of vacuum pressure) until wells are drained (2 - 10 seconds).
10. Add 150 μL of Wash Buffer to each assay well.
11. Apply the plate to the vacuum manifold and vacuum aspirate (do not exceed 10" Hg of vacuum pressure) until wells are drained (2 - 10 seconds).
12. Add 150 μL of Wash Buffer to each assay well. Shake microwell plate on a digital shaker at 500 RPM for 5 minutes to resuspend beads.
13. Begin analyzing samples on a flow cytometer.
Note: It is best to analyze samples on the day of the experiment. Prolonged storage of samples, once the assay is complete, can lead to increased background and reduced sensitivity.
For Tubes:
1. Prepare all reagents as described in previous sections before starting the experiment.
2. Vortex the diluted Capture Beads for at least 5 seconds. Add 50 μL of the diluted Capture Beads to each assay tube.
3. Add 50 μL of Standard or sample to each assay tube.
4. Mix assay tubes gently and incubate for 2 hours at RT.
5. Add 1.0 mL of Wash Buffer to each assay tube and centrifuge at 200 × g for 5 minutes.
6. Carefully aspirate and discard the supernatant from each assay tube leaving approximately 100 μL of liquid in each assay tube. Aspiration should be done as consistently as possible.
7. Add 50 μL of the diluted PE Detection Reagent to each assay tube.
6. Mix assay tubes gently and incubate for 2 hours at RT.
8. Add 1.0 mL of Wash Buffer to each assay tube and centrifuge at 200 × g for 5 minutes.
9. Carefully aspirate and discard the supernatant from each assay tube leaving approximately 100 μL of liquid in each assay tube. Aspiration should be done as consistently as possible.
10. Add 300 μL of Wash Buffer to each assay tube. Vortex assay tubes briefly to resuspend beads.
11. Begin analyzing samples on a flow cytometer. It is recommended that each tube be vortexed briefly before analyzing on the flow cytometer.
Note: It is best to analyze samples on the day of the experiment. Prolonged storage of samples, once the assay is complete, can lead to increased background and reduced sensitivity.
The BD CBA Human TGF-β1 Flex Set must be used in conjunction with a BD CBA Human Soluble Protein Master Buffer Kit (Cat. No. 558264, 100 tests, or 558265, 500 tests), a flow cytometer, and the FCAP Array™ Software (Cat. No. 641488). The top standard point for the BD CBA Human TGF-β1 Flex Set will be 10,000 pg/ml. An example standard curve is shown in Figure 1.
Performance
Limit of Detection: The theoretical limit of detection is 14.9 pg/ml and was determined by evaluating the estimated result of the average MFI of the negative control (0 pg/ml, n=30) + 2 standard deviations.
Product Notices
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- ProClin is a trademark of Rohm and Haas Company.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Warning: CBA lyophilized standard contains 0.02% (w/w) of a CMIT/MIT mixture (3:1), which is a mixture of:5-chloro-2-methyl-4-isothiazolin-3-one [EC No 247-500-7] and 2-methyl-4-isothiazolin-3-one [EC No 220-239-6] (3:1).Hazard statement: May cause an allergic skin reaction.Precautionary statements: Contaminated work clothing should not be allowed out of the workplace. Wear protective gloves/eye/face protection. Wear protective clothing. Avoid breathing mist/vapours/spray. If skin irritation or rash occurs: Get medical advice/attention. IF ON SKIN: Wash with plenty of water. Take off contaminated clothing and wash it before reuse. Dispose of contents/container in accordance with local/regional/national/international regulations.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
| Description | Quantity/Size | Part Number | EntrezGene ID |
|---|---|---|---|
| N/A | 100.0 | N/A | N/A |
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
