-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD Accuri™ C6 Plus Cell Analyzer
- BD FACSAria™ Cell Sorter Cell Sorter
- BD FACSCanto™ Cell Analyzer
- BD FACSDiscover™ A8 Cell Analyzer
- BD FACSDiscover™ S8 Cell Sorter
- BD FACSDuet™ Sample Preparation System
- BD FACSLyric™ Cell Analyzer
- BD FACSMelody™ Cell Sorter
- BD FACSymphony™ Cell Analyzer
- BD LSRFortessa™ Cell Analyzer
- Advanced Training
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Co-staining Cells with Fluorescent Antibodies and BD Oligo-Conjugated Antibodies
Capture of isolated single cells on the BD Rhapsody™ Single-Cell Analysis System often requires initially sorting cells to enrich specific subpopulations and to identify rare cells. Here, we address how to co-stain cells with the same clone of both fluorescent antibodies and oligonucleotide-conjugated antibodies (BD® Single-Cell Multiplexing Kit or BD® AbSeq Ab-Oligos).
Recommendations
- Co-stain cells with fluorescent and oligonucleotide-conjugated antibodies (BD® Single-Cell Multiplexing Kit or BD® AbSeq Ab-Oligos) before cell sorting. This can (i) reduce background noise from unbound BD® AbSeq Antibodies and (ii) minimize cell loss from washing that may be more severe with low cell numbers after sorting.
- Use BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) to resuspend and wash cells.
- If more than two BD Horizon Brilliant™ Fluorescent Antibodies are present in the sorting panel, use BD Horizon™ Brilliant Stain Buffer Plus (Cat. No. 566385) to reduce dye-to-dye interaction.
- If the same antibody clone is used in both fluorescent and oligonucleotide-conjugated versions:
- First, adjust the volume or concentration of the antibodies to ensure that equal microgram (μg) quantities are used
- Second, co-stain cells with these antibodies for 10 minutes on ice, then add the remaining desired antibodies to the cell suspension as described.
- If the specificity for all antibodies used are unique, they can be combined together and cells can be co-stained in a single
step. For each antibody, use the recommended volume per test size (e.g., 20 μL of Sample Tag antibody or 2 μL of BD AbSeq Antibody per 1 million cells) established in other BD protocols. - For BD® AbSeq Antibodies, refer to Single Cell Labeling with BD® AbSeq Ab-Oligos
- For Sample Tag antibodies (1-plex to 40-plex), refer to Single Cell Labeling with the BD® Single-Cell Multiplexing Kits and AbSeq Ab-Oligos
- For Sample Tag antibodies (41-plex to 100-plex), refer to Single-Cell Labeling with BD® Single-Cell Multiplexing Kit and BD® AbSeq Ab-Oligos
- For fluorescent antibodies, visit us online at bdbiosciences.com Note: Cell staining protocols are optimized for up to 1 million cells. Higher number of cells used may result in lower binding of antibodies.
- The final staining volume of all antibodies and cell suspension should be 200 μL to ensure best performance of BD® AbSeq Ab-Oligos and BD® Single-Cell Multiplexing Kit.
Co-Staining Workflows
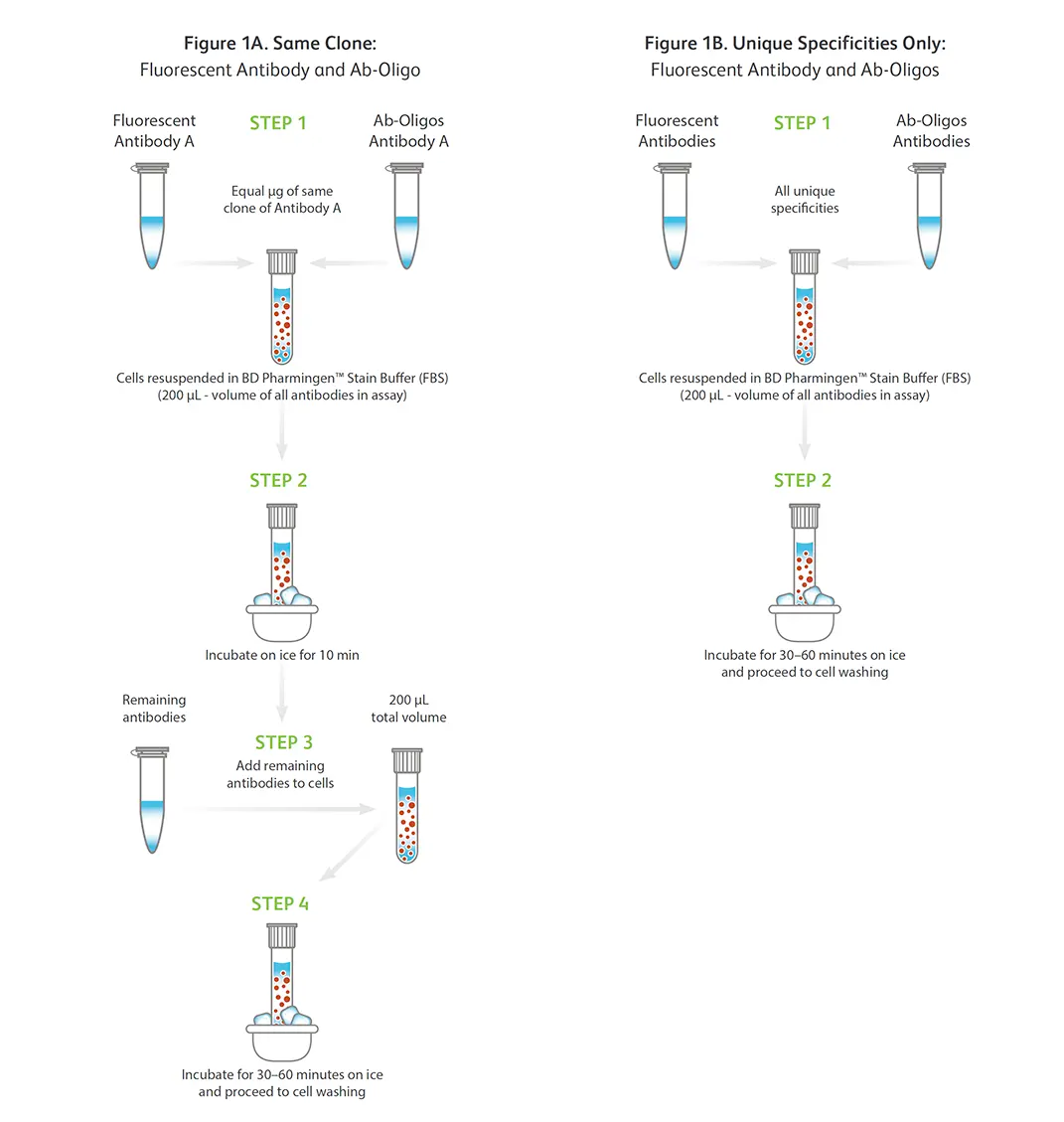
A. Same-clone antibody co-staining:
(Step 1) To cells suspended in BD Pharmingen™ Stain Buffer (FBS), add equal µg amounts of fluorescent and oligonucleotideconjugated antibodies (BD® AbSeq Ab-Oligos or BD® Single-Cell Multiplexing Kit) that share the same clone.
(Step 2) Incubate antibodies and cell mixture on ice for 10 minutes. (Step 3) Add the remaining fluorescent and oligonucleotide-conjugated antibodies to the mixture for a final volume of 200 µL. (Step 4) Incubate entire antibody and cell mixture on ice for 30 to 60 minutes and then proceed to cell washing.
B. Co-staining cells with antibodies having unique specificities:
(Step 1) In a single step, combine all fluorescent and oligonucleotide-conjugated antibodies (BD AbSeq Ab-Oligos or BD® Single-Cell Multiplexing Kit) that have unique specificities together with cells suspended in BD Pharmingen™ Stain Buffer (FBS) for a final volume of 200 µL.
(Step 2) Incubate entire antibody and cell mixture on ice for 30 to 60 minutes and then proceed to cell washing.
Co-Staining Procedure
- If there are no overlapping specificities between the fluorescent and oligonucleotide-conjugated antibodies, proceed to step 3.
- If using the same clone in both fluorescent and oligonucleotide-conjugated versions of the antibody:
a. Determine the concentration of the fluorescent antibody and the oligonucleotide-conjugated antibody using the following:
i. For the fluorescent antibody, visit us online at regdocs.bd.com/regdocs/qcinfo. Use the catalog number and lot number to find the correct concentration
ii. For the oligonucleotide-tagged antibody, contact BD customer support at scomix@bdscomix.bd.com.
b. Determine the appropriate amount of the fluorescent antibody to use so it equals the μg quantity of the oligonucleotide-conjugated antibody. - Calculate the total volume of all antibodies to be used in the assay, including adjusted volumes of the same clone from step 2 (if applicable) and volumes for antibodies with different specificities.
- Subtract the value calculated in step 3 from 200 μL to obtain the volume of BD Pharmingen™ Stain Buffer (FBS) that will be used during staining. (See Table 1 below)
- Suspend cells in the volume of BD Pharmingen™ Stain Buffer (FBS) calculated in step 4.
Note: For samples containing myeloid and B lymphocytes, BD recommends blocking non-specific Fc Receptor mediated false-positive with BD Fc Block™ Buffer (human cell, Cat. No 564220; mouse cell, Cat. No. 553142) before staining. For human cells, replace 5 μL of stain buffer with human BD Fc Block™ Buffer and incubate cells for 10 minutes at room temperature. For mouse cells, place 2 uL of stain buffer with mouse BD Fc Block™ Buffer and incubate cells for 5 minutes at 4 °C. - If you are not using a shared clone, proceed directly to step 7. If using a shared clone:
a. Add the appropriate volume of the shared-clone for the fluorescent and oligonucleotide-conjugated antibodies (BD® Single-Cell Multiplexing Kit or BD AbSeq Ab-Oligos).
b. Pipet-mix and incubate on ice for 10 minutes. - Add the remaining fluorescent and/or BD AbSeq Antibodies to attain a final volume of 200 μL.
- Pipet-mix and incubate the mixture for 30–60 minutes on ice.
- Transfer labeled cell suspension to a 5 mL polystyrene Falcon™ Tube (Corning™ Cat. No. 352054) if cells are in a different tube type.
- Add 2 mL BD Pharmingen™ Stain Buffer (FBS) to labeled cells and pipet-mix.
- Centrifuge tube at 400 x g for 5 minutes.
- Decant supernatant and keep tube inverted. Gently blot inverted tube on lint-free wipe to remove residual liquid from the rim of the tube.
Note: Do not tilt and decant the tube multiple times to get rid of fluid as this may dislodge cells from the tube. - Repeat steps 10–12 once or twice more for a total of 2 or 3 washes.
- Resuspend cell pellet in BD FACS™ Pre-Sort Buffer (Cat. No. 563503) and proceed with flow-sorting workflow.
- After sorting, proceed with the protocol outlined in Single Cell Capture and cDNA Synthesis with the BD Rhapsody Single-Cell Analysis System.
| Component | Volume for one sample |
|---|---|
All antibodies | 55 μL (10 μL + 25 μL + 20 μL) |
Shared clone of fluorescent and BD® AbSeq Ab-Oligo (equal μg quantities) | 10 μL (8 μL flow antibody + 2 μL BD AbSeq Ab-Oligos) |
5-plex fluorescent antibodies (unique specificities) | 25 μL (5 x 5 μL) |
10-plex BD® AbSeq Antibodies (unique specificities) | 20 μL (10 x 2 μL) |
Cells suspended in BD Pharmingen™ Stain Buffer (FBS) | 145 μL (200–55 μL) |
Total Volume | 200 μL |
Table 1. Cell labeling example
In this example, cells are stained with same-clone fluorescent and Ab-oligo antibodies, a 5-plex fluorescent antibody panel and a 10-plex BD® AbSeq Antibody panel.
Note: The antibody pool should be made with 30% overage to ensure the correct amount is added for staining.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
BD Life Sciences, San Jose, CA, 95131, USA
bdbiosciences.com
BD, the BD Logo, FACS, Fc Block, Horizon, Horizon Brilliant, Pharmingen and Rhapsody are trademarks of Becton, Dickinson and Company or its affiliates. All other trademarks are the property of their respective owners. © 2021 BD. All rights reserved.