-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




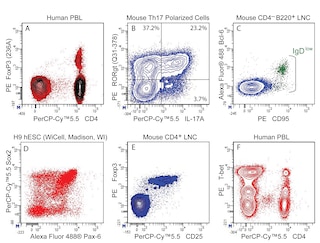
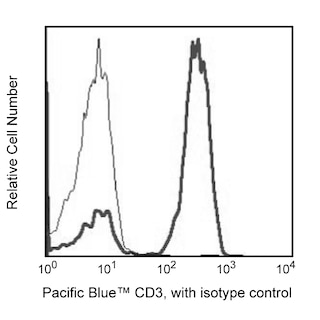
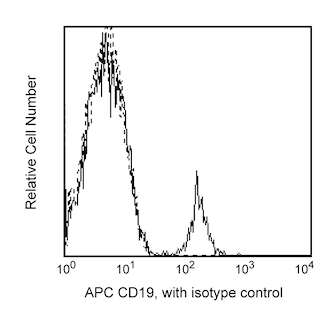
Multiparameter Analyses of Oct-2 Expression: Panel 1: Human peripheral blood mononuclear cells, PBMCs, were fixed and permeabilized with BD Pharmingen™ Transcription Factor Buffer Set. Cells were then stained with Purified Rat IgG2a, κ Isotype Control or Purified Rat Anti-Oct-2 and followed with PE Mouse Anti-Rat IgG2a. Prior to fixation, PBMCs were stained with Pacific Blue™ Anti-Human CD3, APC Anti-Human CD19 and FITC Anti-Human CD14. The target event acquisition stop count was 5,000 CD19+ B cells. Two-color flow cytometric data were derived from gated events with the light-scatter characteristics and positive staining for CD3+ T cells (labeled T), CD14+ monocytes (M) or CD19+ B lymphocytes (B). Flow cytometry was performed using a BD LSRFortessa™ Cell Analyzer. Panels 2a, 2b: BALB/c mouse splenocytes and lymph node cells were similarly fixed, permeabilized and stained for Oct-2 as described. After washing, cells were stained with Alexa Fluor® 647 Anti-Mouse CD45R/B220 and FITC Anti-Mouse CD3e and analyzed. Panel 3a: Western blot of Oct-2 expressed by human (Raji, HL-60, or PBMC) or mouse (thymus, spleen) cells. Lysates (20 μg protein/lane) were blotted using Anti-Oct-2, HRP Anti-Rat Ig (Southern Biotech, 3030-05) followed by development with Pierce HRP Substrate (Cat. No. 34080). Oct-2 was identified as a protein band of ~50-60 kDa. Panel 3b: Immunohistochemical analysis of tonsillar Oct-2 expression. Paraffin-embedded tissue sections (5 μm) on poly-l-lysine treated slides were dewaxed, rehydrated and heated (3 min) in TRIS-EDTA 0.01M (pH 9) to retrieve antigen. After cooling, slides were placed in TBS (5 min). Endogenous peroxidase was blocked with a peroxidase-block reagent (DAKO, Denmark). Slides were incubated (30 min, RT) with Anti-Oct-2 antibody. Binding was detected by the peroxidase-based EnVision+ method and sections were stained with hematoxylin. Data was reproduced with the permission of The Walter and Eliza Hall Institute of Medical Research.


BD Pharmingen™ Purified Rat Anti-Oct-2

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Sodium azide is a reversible inhibitor of oxidative metabolism; therefore, antibody preparations containing this preservative agent must not be used in cell cultures nor injected into animals. Sodium azide may be removed by washing stained cells or plate-bound antibody or dialyzing soluble antibody in sodium azide-free buffer. Since endotoxin may also affect the results of functional studies, we recommend the NA/LE (No Azide/Low Endotoxin) antibody format, if available, for in vitro and in vivo use.
- All other brands are trademarks of their respective owners.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- Pacific Blue™ is a trademark of Molecular Probes, Inc., Eugene, OR.
Companion Products

.png?imwidth=320)
The 9A2 monoclonal antibody specifically binds to Oct-2 that has a molecular weight of approximately 50-60 kDa. The 9A2 antibody was generated against the 44 N-terminal amino acids common to mouse Oct-2 isoforms and crossreacts with both human and mouse Oct-2. Oct-2 is a POU class 2 homeobox 2 transcription factors (POU2F2) that is also known as OCT2, OTF2, Lymphoid-restricted immunoglobulin octamer-binding protein NF-A2 and Octamer-binding transcription factor 2. The POU domain in the Oct-2 protein is required for low and high affinity cooperative binding of the octomer sequence and heptamer site in immunoglobulin promoters and promoters for other genes. Oct-2 is expressed in B cells, activated CD4+ and CD8+ T cells, monocytes/macrophages and subsets of dendritic cells. Oct-2 is reportedly associated with neoplasms including Diffuse Large B-cell lymphoma and Nodular Lymphocyte predominant Hodgkin's lymphoma. Studies with knockout mouse models suggest Oct-2 is required for B cell maturation, but not for B cell commitment, and for B cell production of IL-6.
Development References (8)
-
Borestrom C, Forsman A, Ruetschi U, Rymo L. E2F1, ARID3A/Bright and Oct-2 factors bind to the Epstein-Barr virus C promoter, EBNA1 and oriP, participating in long-distance promoter-enhancer interactions. J Gen Virol. 2012; 93(Ot 5):1065-1075. (Biology). View Reference
-
Browne P, Petrosyan K, Hernandez A, Chan JA. The B-cell transcription factors BSAP, Oct-2, and BOB.1 and the pan-B-cell markers CD20, CD22, and CD79a are useful in the differential diagnosis of classic Hodgkin lymphoma. Am J Clin Pathol. 2003; 120(5):767-777. (Biology). View Reference
-
Chou YY, Gao JI, Chang SF, Chang PY, Lu SC. Rapamycin inhibits lipopolysaccharide induction of granulocyte-colony stimulating factor and inducible nitric oxide synthase expression in macrophages by reducing the levels of octamer-binding factor-2. FEBS J. 2011; 278(1):85-96. (Biology). View Reference
-
Corcoran LM, Koentgen F, Dietrich W, Veale M, Humbert PO. All known in vivo functions of the Oct-2 transcription factor require the C-terminal protein domain. J Immunol. 2004; 172(5):2962-2969. (Immunogen: Gel shift, Western blot). View Reference
-
Herbeck R, Teodorescu Brinzeu D, Giubelan M, Lazar E, Dema A, Ionita H. B-cell transcription factors Pax-5, Oct-2, BOB.1, Bcl-6, and MUM1 are useful markers for the diagnosis of nodular lymphocyte predominant Hodgkin lymphoma. Rom J Morphol Embryol. 2011; 52(1):69-74. (Biology). View Reference
-
Kang SM, Tsang W, Doll S, et al. Induction of the POU domain transcription factor Oct-2 during T-cell activation by cognate antigen. Mol Cell Biol. 1992; 12(7):3149-3154. (Biology). View Reference
-
Karnowski A, Chevrier S, Belz GT, et al. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 2012. (Biology: Gel shift, Western blot). View Reference
-
Saglam A, Uner AH. Immunohistochemical expression of Mum-1, Oct-2 and Bcl-6 in systemic anaplastic large cell lymphomas. Tumori. 2011; 97(5):634-638. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.