-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Vacutainer® CPT™ Mononuclear Cell Preparation Tube - Sodium Citrate
(IVD)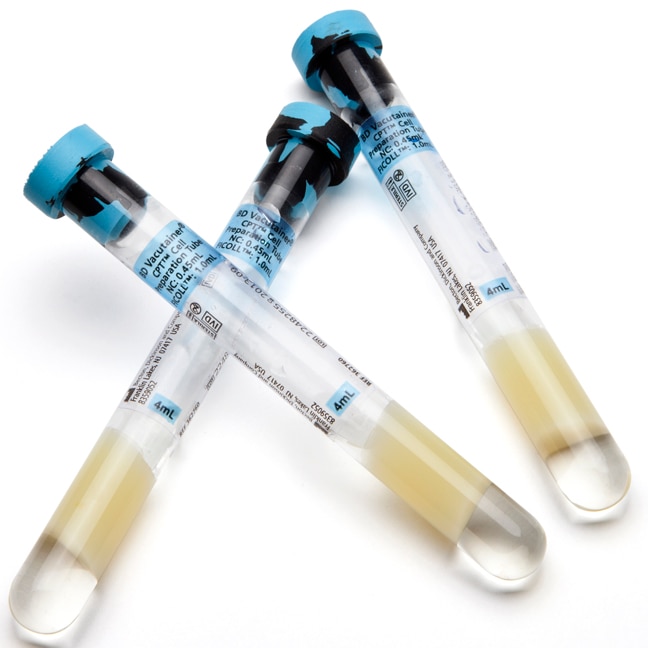

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
BD Vacutainer® CPT™ is a fully-closed system for separation of mononuclear cells from whole blood, where cell separation is carried out in the primary blood collection tube. This decreases the complexity of steps for mononuclear cell separation, thereby minimizing variability from sample processing.
CPT™ is an evacuated, sterile blood collection tube containing buffered sodium citrate or sodium heparin anticoagulant, liquid density medium and an inert gel barrier. It is intended for the collection of whole blood and the subsequent separation of mononuclear blood cells for the purposes of in vitro diagnostic examination.
Whole blood is drawn directly into the CPT™ using standard phlebotomy techniques and processed in the same tube. During centrifugation, the gel forms a physical barrier between the mononuclear cells in plasma and the erythrocytes and granulocytes. The separated, concentrated suspension of mononuclear cells in plasma can then be transported in the primary blood collection tube.
| Tube Material | Glass |
| Tube Size | 13x100 mm |
| Draw Volume | 4 mL |
| Closure Type | Conventional Stopper |
| Closure Color | Blue / Black |
| Label | Mylar (transparent) |
| Anticoagulant | Sodium Citrate |
| Additive | Ficoll™ Hypaque™ Solution |
| Sterilization | Autoclave , 10-6 SAL , ISO17665-1 |
Preparation And Storage
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.