-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
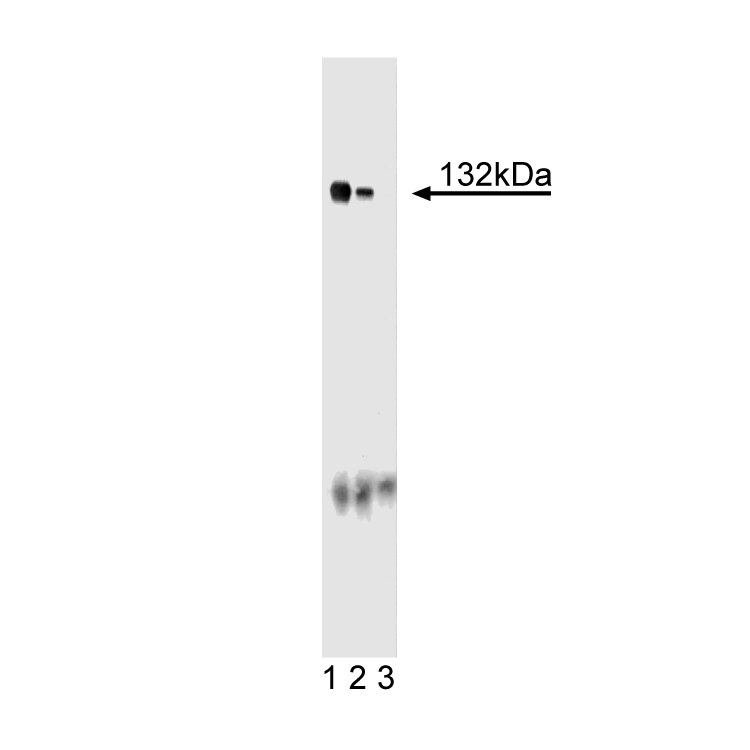




Western blot analysis of Ceruloplasmin on a rat testis lysate. Lane 1: 1:250, lane 2: 1:500, lane 3: 1:1000 dilution of the anti-Ceruloplasmin antibody.

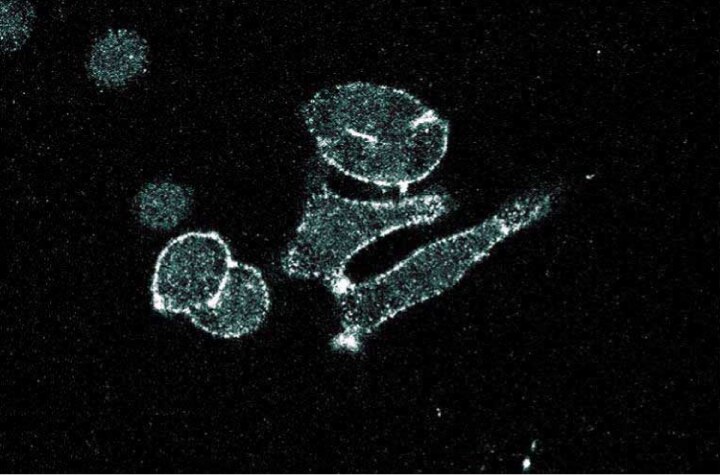
Immunofluorescence staining of SK-BR-3 cells (Human breast adenocarcinoma; ATCC HTB-30).


BD Transduction Laboratories™ Purified Mouse Anti-Ceruloplasmin

BD Transduction Laboratories™ Purified Mouse Anti-Ceruloplasmin

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Western blot: Please refer to http://www.bdbiosciences.com/pharmingen/protocols/Western_Blotting.shtml
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
Companion Products

.png?imwidth=320)
Ceruloplasmin (CP) is a copper-containing glycoprotein that carries approximately 90% of plasma copper. CP contains six copper atom binding sites and is prone to transfer its copper atoms to tissues, however it is not essential for copper transport. In cultured endothelial cells, CP release of copper in the cytosol inhibits nitric oxide synthase activation, and this may be important for regulating vascular tone in blood vessels. In addition to its copper binding role, CP has also been shown to have ferroxidase activity, which involves conversion of ferrous to ferric iron. The iron metabolism disorder aceruloplasminemia results from mutations in CP and is characterized by massive iron deposits in liver, pancreas, brain, retina, and other tissues. CP is expressed in the liver, the splenic reticuloendothelial system, the bronchiolar epithelium of the lung, and the retina and brain. The cell-specific expression of CP in neural tissues may account for the neuropathologies associated with aceruloplaminemia, while the wide expression pattern of CP may demonstrate its importance in the regulation of copper and iron levels in many tissues.
This antibody is routinely tested by western blot analysis. Other applications were tested at BD Biosciences Pharmingen during antibody development only or reported in the literature.
Development References (5)
-
Attieh ZK, Mukhopadhyay CK, Seshadri V, Tripoulas NA, Fox PL. Ceruloplasmin ferroxidase activity stimulates cellular iron uptake by a trivalent cation-specific transport mechanism. J Biol Chem. 1999; 274(2):1116-1123. (Biology). View Reference
-
Bianchini A, Musci G, Calabrese L. Inhibition of endothelial nitric-oxide synthase by ceruloplasmin. J Biol Chem. 1999; 274(29):20265-20270. (Biology). View Reference
-
Kaplan J, O'Halloran TV. Iron metabolism in eukaryotes: Mars and Venus at it again. Science. 1996; 271(5255):1510-1512. (Biology). View Reference
-
Klomp LW, Farhangrazi ZS, Dugan LL, Gitlin JD. Ceruloplasmin gene expression in the murine central nervous system. J Clin Invest. 1996; 98(1):207-215. (Biology). View Reference
-
Mukhopadhyay CK, Attieh ZK, Fox PL. Role of ceruloplasmin in cellular iron uptake. Science. 1998; 279(5351):714-717. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.