-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
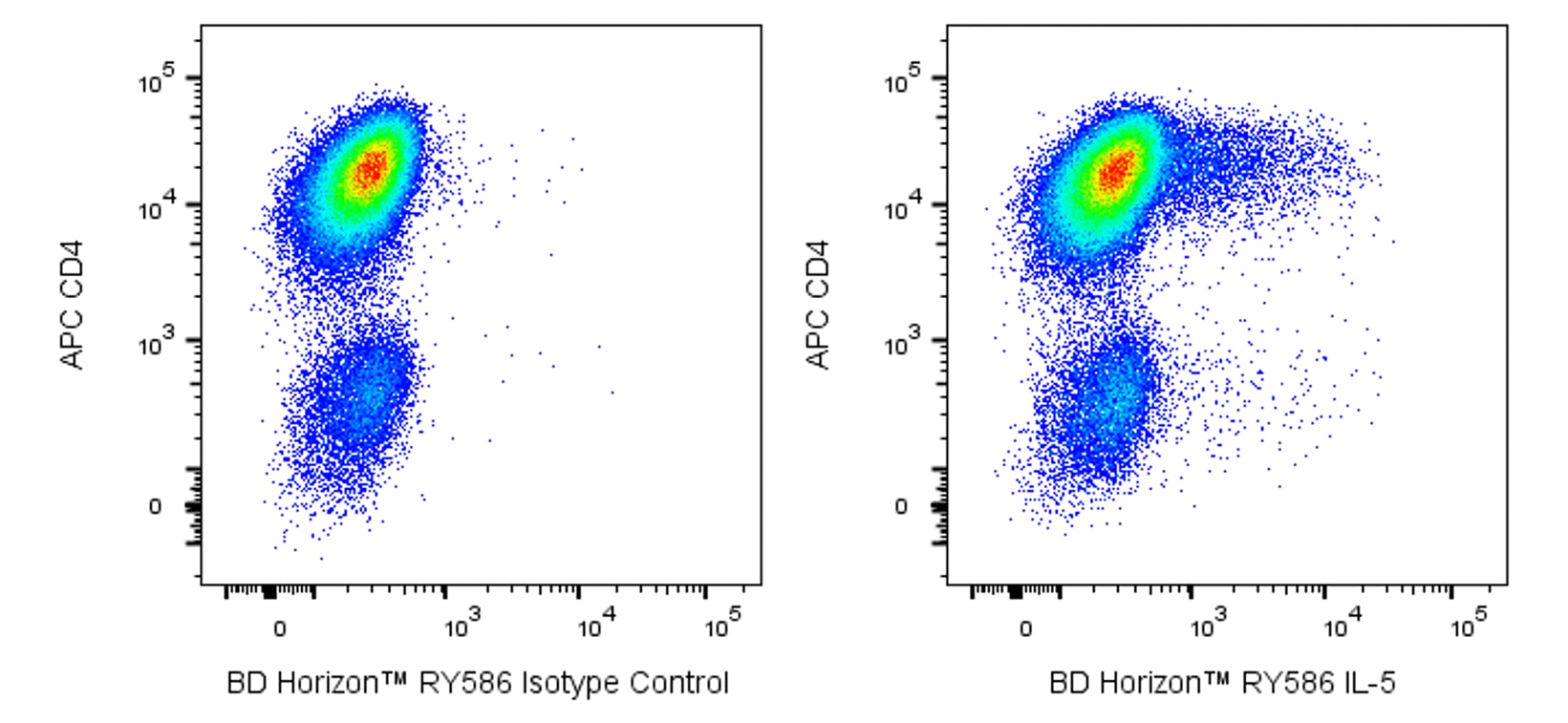



Two-color flow cytometric analysis of IL-5 expression in stimulated Mouse T cells. Mouse splenic CD4+ T cells were cultured (2 days) with plate-bound Purified NA/LE Hamster Anti-Mouse CD3e (Cat. No. 553057; 10 μg/ml for coating) and soluble Purified NA/LE Hamster Anti-Mouse CD28 (Cat. No. 553294; 2 μg/ml) antibodies with Recombinant Mouse IL-2 (Cat. No. 550069; 10 ng/ml) and Mouse IL-4 (Cat. No. 550067; 25 ng/ml) proteins. The cells were subsequently cultured (3 days) with Recombinant IL-2 and IL-4. The cells were then stimulated with Phorbol 12-Myristate 13-Acetate (PMA; Sigma Cat. No. P-8139) and Ionomycin (Sigma Cat. No. I-0634) in the presence of BD GolgiStop™ Protein Transport Inhibitor (containing Monensin) (Cat. No. 554724) for 6 hours. The cells were washed and fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655). The fixed cells were washed and then permeabilized and stained in BD Perm/Wash™ Buffer (Cat. No. 554723) with APC Rat Anti-Mouse CD4 antibody (Cat. No. 561091/553051) and with either BD Horizon™ RY586 Rat IgG1, κ Isotype Control (Cat. No. 568822; Left Plot) or BD Horizon™ RY586 Rat Anti-Mouse/Anti-Human IL-5 antibody (Cat. No. 568530/568531; Right Plot) at 0.125 µg/test using the BD Biosciences Intracellular Cytokine Staining protocol. The bivariate pseudocolor density plot showing the correlated expression of IL-5 (or Ig Isotype control staining) versus CD4 was derived from gated events with the forward and side light-scatter characteristics of intact stimulated lymphocytes. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software. Data shown on this Technical Data Sheet are not lot specific.


BD Horizon™ RY586 Rat Anti-Mouse/Anti-Human IL-5

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- CF™ is a trademark of Biotium, Inc.
Companion Products





The TRFK5 antibody reacts with mouse interleukin-5 (IL-5) and cross-reacts with human IL-5. The TRFK5 antibody has been reported to cross react with IL-5 from rhesus monkey. This is a neutralizing antibody.
Development References (11)
-
Abrams J. Immunoenzymetric assay of mouse and human cytokines using NIP-labeled anti-cytokine antibodies. Curr Protoc Immunol. 2001; 1:6.20-6.21. (Clone-specific: ELISA). View Reference
-
Abrams JS, Roncarolo MG, Yssel H, Andersson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992; 127:5-24. (Clone-specific: ELISA, Neutralization). View Reference
-
Assenmacher M, Schmitz J, Radbruch A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-gamma and in interleukin-4-expressing cells. Eur J Immunol. 1994; 24(5):1097-1101. (Clone-specific: Flow cytometry). View Reference
-
Hobbs MV, Weigle WO, Noonan DJ, et al. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993; 150(8):3602-3614. (Clone-specific: ELISA). View Reference
-
Mo XY, Sarawar SR, Doherty PC. Induction of cytokines in mice with parainfluenza pneumonia. J Virol. 1995; 69(2):1288-1291. (Clone-specific: ELISA). View Reference
-
Pala P, Hussell T, Openshaw PJ. Flow cytometric measurement of intracellular cytokines.. J Immunol Methods. 2000; 243(1-2):107-24. (Clone-specific: Flow cytometry). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology: Blocking, Flow cytometry). View Reference
-
Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991; 119:65-93. (Clone-specific: ELISA). View Reference
-
Sarawar SR, Sangster M, Coffman RL, Doherty PC. Administration of anti-IFN-gamma antibody to beta 2-microglobulin-deficient mice delays influenza virus clearance but does not switch the response to a T helper cell 2 phenotype. J Immunol. 1994; 153(3):1246-1253. (Clone-specific: ELISA). View Reference
-
Schumacher JH, O'Garra A, Shrader B, et al. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. J Immunol. 1988; 141(5):1576-1581. (Clone-specific: ELISA, Neutralization). View Reference
-
Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990; 145(11):3796-3806. (Clone-specific: Neutralization). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.