-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- Advanced Training
-
- BD FACSDiscover™ S8 Cell Sorter Product Training
- Accuri C6 Plus Product-Based Training
- FACSAria Product Based Training
- FACSCanto Product-Based Training
- FACSLyric Product-Based Training
- FACSMelody Product-Based Training
- FACSymphony Product-Based Training
- HTS Product-Based Training
- LSRFortessa Product-Based Training
- United States (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
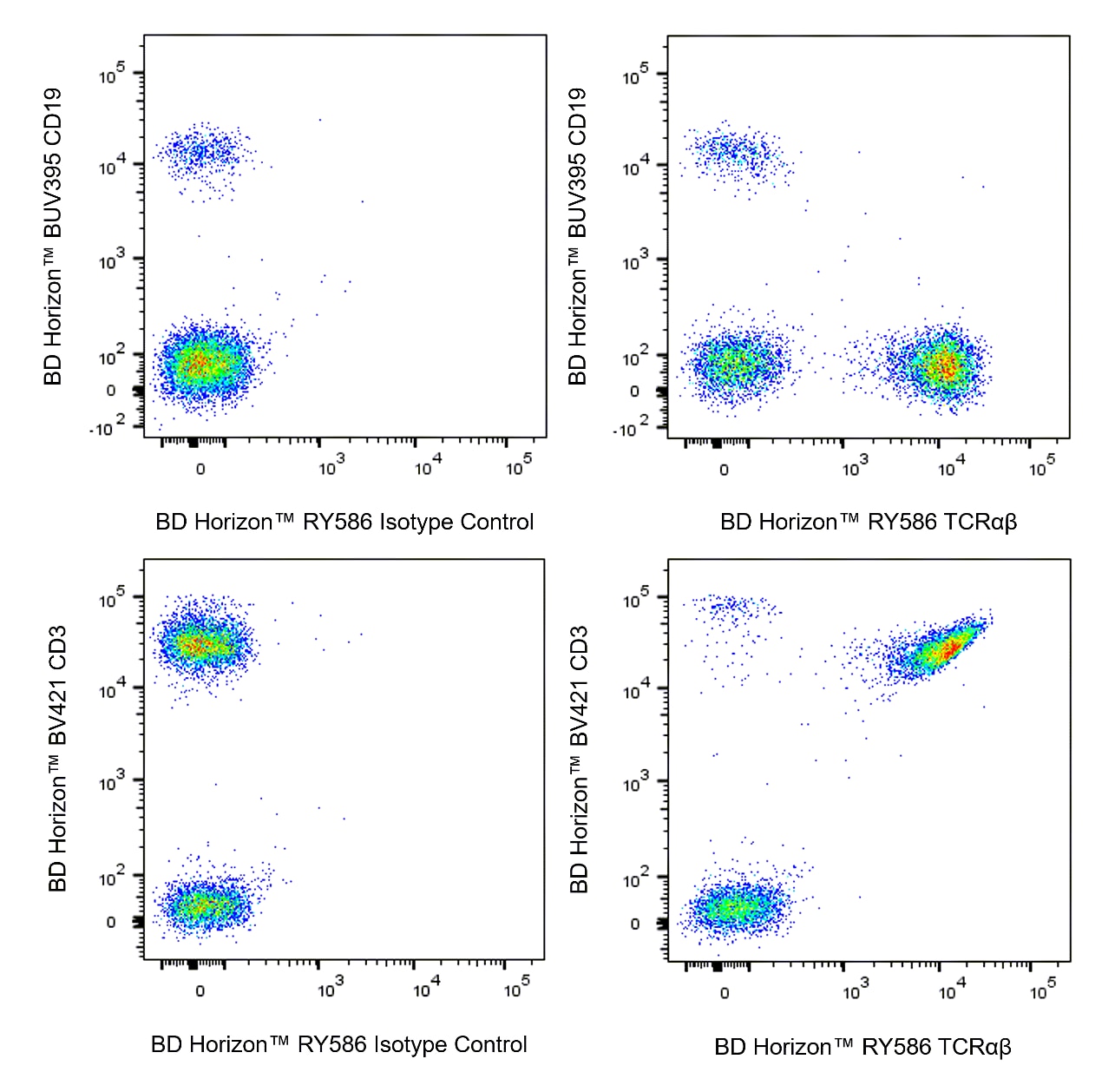



Multicolor flow cytometric analysis of TCRαβ expression on Human peripheral blood lymphocytes. Human whole blood was stained with BD Horizon™ BUV395 Mouse Anti-Human CD19 antibody (Cat. No. 563549/563551), BD Horizon™ BV421 Mouse Anti-Human CD3 antibody (Cat. No. 562426/562427) and either BD Horizon™ RY586 Mouse IgG1, κ Isotype Control (Cat No. 568097; Left Plots) or BD Horizon™ RY586 Mouse Anti-Human TCRαβ antibody (Cat. No. 568516/568517; Right Plots). Erythrocytes were lysed with BD FACS Lysing™ Solution (Cat. No. 349202). Flow cytometry and data analysis were performed using a LSRFortessa™ X-20 Flow Cytometer System and FlowJo™ software. Upper Plots: Bivariate pseudocolor density plots showing the correlated expression of TCRαβ (or Ig Isotype control staining) versus CD19 were derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Bottom Plots: Bivariate pseudocolor density plots showing the correlated expression of TCRαβ (or Ig Isotype control staining) versus CD3 were derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes.


BD Horizon™ RY586 Mouse Anti-Human TCRαβ

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- CF™ is a trademark of Biotium, Inc.
Companion Products



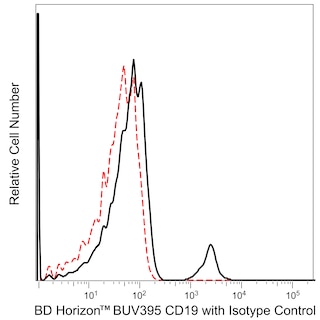


The IP26 monoclonal antibody specifically recognizes a monomorphic determinant on the αβ T-cell receptor (TCRαβ). The TCRαβ is a disulfide-linked 80 kDa heterodimer consisting of a 44 kDa α chain and a 37 kDa β chain. The TCRαβ is normally expressed on 50-70% of thymocytes and on a large fraction of mature T cells including greater than 95% of peripheral blood CD3+ T cells. The TCRαβ serves as a receptor for peptide antigens bound to MHC molecules. The IP26 antibody is mitogenic and useful for the flow cytometric analysis of TCRαβ+ T-cell subsets.
Development References (5)
-
Agrawal SG, Marquet J, Plumas J, et al. Multiple co-stimulatory signals are required for triggering proliferation of T cells from human secondary lymphoid tissue. Int Immunol. 2001; 13(4):441-450. (Clone-specific: Activation, Functional assay, Stimulation). View Reference
-
Nikolova M, Marie-Cardine A, Boumsell L, Bensussan A. BY55/CD160 acts as a co-receptor in TCR signal transduction of a human circulating cytotoxic effector T lymphocyte subset lacking CD28 expression. Int Immunol. 2002; 14(5):445-451. (Clone-specific: Flow cytometry). View Reference
-
Oettgen HC, Kappler J, Tax WJ, Terhorst C. Characterization of the two heavy chains of the T3 complex on the surface of human T lymphocytes. J Biol Chem. 1984; 259(19):12039-12048. (Biology). View Reference
-
Ortonne N, Huet D, Gaudez C, et al. Significance of circulating T-cell clones in Sezary syndrome. Blood. 2006; 107(10):4030-4038. (Clone-specific: Flow cytometry, Fluorescence activated cell sorting). View Reference
-
van Dongen JJ, Lhermitte L, Böttcher S, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012; 26(9):1908-1975. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.