Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

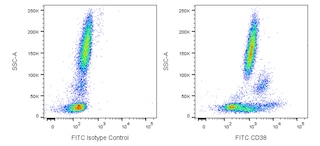
Multiparameter flow cytometric analysis of CD38 expression on human peripheral blood leucocyte populations. Whole blood was stained with either FITC Mouse IgG1, κ Isotype Control (Cat. No. 554679; Left Plot) or FITC Mouse Anti-Human CD38 antibody (Cat. No. 567147/567148; Right Plot). Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). A bivariate pseudocolor density plot showing the correlated expression of CD38 (or Ig Isotype control staining) versus side-light scatter (SSC-A) signals was derived from gated events with the forward and side-light scatter characteristics of intact leucocyte populations. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.
.png)

BD Pharmingen™ FITC Mouse Anti-Human CD38
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD™ CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cell and CompBead to ensure that BD Comp beads are appropriate for your specific cellular application.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
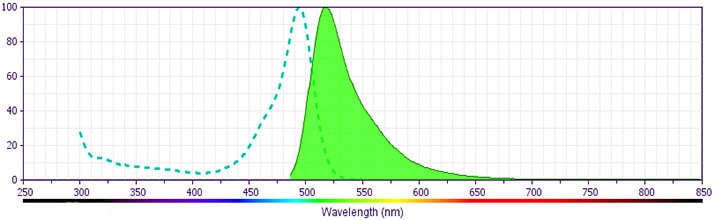
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products


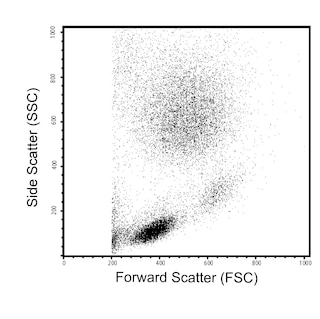

The HB7 monoclonal antibody specifically binds to human CD38. CD38 is a type II transmembrane glycoprotein of 45 kDa with a protein core of 35 kDa. The CD38 antigen is expressed on essentially all pre-B lymphocytes, plasma cells, and thymocytes. It is also present on activated T lymphocytes, natural killer (NK) lymphocytes, myeloblasts, and erythroblasts. The antigen is expressed during the early stages of T- and B-lymphocyte differentiation, is lost during the intermediate stages of maturation, and then reappears during the final stages of maturation. The CD38 antigen is expressed on 90% of CD34+ cells, and is not expressed on pluripotent stem cells. Coexpression of CD38 antigen on CD34+ cells indicates lineage commitment of those cells. CD38 is a counter-receptor of CD31. It is also expressed in T- and B-acute lymphoblastic leukemia (ALL), Burkitt's lymphoma, multiple myeloma, acute myeloid leukemia (AML), and chronic lymphocytic leukemia (CLL).
Development References (15)
-
Deaglio S, Morra M, Mallone R, et al. Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. J Immunol. 1998; 160(1):395-402. (Biology). View Reference
-
Dörken B, Möller P, Pezzutto A, Schwartz-Albiez R, Moldenhauer G. B-cell antigens: CD38. In: Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:86.
-
Ghia P, Guida G, Stella S, et al. The pattern of CD38 expression defines a distinct subset of chronic lymphocytic leukemia (CLL) patients at risk of disease progression. Blood. 2003; 101(4):1262-1269. (Clone-specific: Flow cytometry). View Reference
-
Giorgi JV. Lymphocyte subset measurements: significance in clinical medicine. In: Rose NR, Friedman H, Fahey JL, ed. Manual of Clinical Laboratory Immunology. 3rd ed.. Washington, DC: American Society for Microbiology; 1986:236-246.
-
Landay A, Ohlsson-Wilhelm B, Giorgi JV. Application of flow cytometry to the study of HIV infection. AIDS. 1990; 4(6):479-497. (Biology). View Reference
-
Ling NR, Maclennan ICM, Mason DY.. B-cell and plasma cell antigens: new and previously defined clusters. In: McMichael AJ. A.J. McMichael .. et al., ed. Leucocyte typing III : white cell differentiation antigens. Oxford New York: Oxford University Press; 1987:302-335.
-
Nicholson JKA, Jones BM. Immunophenotyping by flow cytometry: its use in HIV infection. Labmedica. 1989; 6:21-26. (Biology).
-
Pezzutto A, Behm F, Callard RE. Flow cytometry analysis of the B-cell blind panel: joint report. In: Knapp W. W. Knapp .. et al., ed. Leucocyte typing IV : white cell differentiation antigens. Oxford New York: Oxford University Press; 1989:165-174.
-
Reinherz EL, Kung PC, Goldstein G, Levey RH, Schlossman SF. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980; 77(3):1588-1592. (Biology). View Reference
-
Salazar-Gonzalez JF, Moody DJ, Giorgi JV, Martinez-Maza O, Mitsuyasu RT, Fahey JL. Reduced ecto-5'-nucleotidase activity and enhanced OKT10 and HLA-DR expression on CD8 (T suppressor/cytotoxic) lymphocytes in the acquired immune deficiency syndrome: evidence of CD8 cell immaturity. J Immunol. 1985; 135(3):1778-1785. (Biology). View Reference
-
Tedder TF, Clement LT, Cooper MD. Discontinuous expression of a membrane antigen (HB-7) during B lymphocyte differentiation. Tissue Antigens. 1984; 24(3):140-149. (Immunogen: Flow cytometry, Immunofluorescence, Immunoprecipitation). View Reference
-
Tedder TF, Crain MJ, Kubagawa H, Clement LT, Cooper MD. Evaluation of lymphocyte differentiation in primary and secondary immunodeficiency diseases. J Immunol. 1985; 135(3):1785-1791. (Clone-specific: Immunofluorescence). View Reference
-
Terstappen LW, Hollander Z, Meiners H, Loken MR. Quantitative comparison of myeloid antigens on five lineages of mature peripheral blood cells. J Leukoc Biol. 1990; 48(2):138-148. (Biology). View Reference
-
Terstappen LW, Huang S, Picker LJ. Flow cytometric assessment of human T-cell differentiation in thymus and bone marrow. Blood. 1992; 79(3):666-677. (Biology). View Reference
-
Terstappen LW, Huang S, Safford M, Lansdorp PM, Loken MR. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38- progenitor cells. Blood. 1991; 77(6):1218-1227. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.