-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Rheumatology
Rheumatic diseases, such as rheumatoid arthritis (RA), lupus and multiple sclerosis, are caused by autoimmune responses. While in immune deficiency disorders the immune system fails to elicit the appropriate immune responses, in autoimmune disorders the immune system overresponds against one’s own antigens. This failure to distinguish between self and nonself arises from breach of immune tolerance, the preventative mechanisms the immune system has in place to prevent attacking itself.1 Autoimmunity could affect specific organs (RA) or they could be systemic (lupus). Some diseases such as ankylosing spondylosis are considered both autoimmune as well as inflammatory arthritic diseases.
Examples of rheumatic diseases
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by inflammation and damage of the joints throughout the body, including hands and feet and affects about 0.5–1% of the population, being more common among women than men in the United States.2 Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease. It affects multiple organ systems, such as the skin, kidneys, lungs and the central nervous system, by producing autoantibodies.3 Ankylosing spondylosis (AS) is a chronic, progressive inflammatory rheumatic disease of the axial musculoskeletal system caused by multiple genes.4
Genetic basis of AS
Genetic factors contribute significantly to rheumatological disease. In particular, the human leukocyte antigen (HLA) locus accounts for about 50% of genetic predisposition to rheumatological disease, with strong involvement of HLA-DRB1, a major histocompatibility complex (MHC) class II molecule.4 Other non-HLA susceptibility genes, such as protein-arginine deiminase type 4 (PADI4) and interleukin-2 receptor subunit α, are also implicated in rheumatological disease. HLA-B27, an MHC class I molecule, has a strong association with AS. HLA-B27 testing is routinely used to screen for ankylosing spondylitis.5,6
BD Biosciences tool for rheumatic disorders
BD Biosciences provides a tool for screening the presence of HLA-B27 antigen on lymphocyte surfaces. The presence of HLA-B27 is strongly associated with ankylosing spondylitis, a rheumatic disorder. The BD® HLA-B27 Kit offers rapid detection of HLA-B27 antigen expression in erythrocyte-lysed whole blood using BD flow cytometry systems.
Precision Table
| Precision | SD of LMF |
|---|---|
| Within Run | 0.62 |
| Between Runs | 0.99 |
| Between Days | 0.54 |
| System Total | 1.18 |
The precision of the BD FACSCanto™ II System was estimated using ten samples, five positive samples and five negative samples, for the HLA-B27 antigen. Samples were run in duplicate for two days, two runs each day, using three BD FACSCanto™ II instruments and three operators. The standard deviation (SD) for the mean of the values for HLA-B27 FITC log median fluorescence (LMF) for each of the variables was calculated.
Cross-reactivity characterization
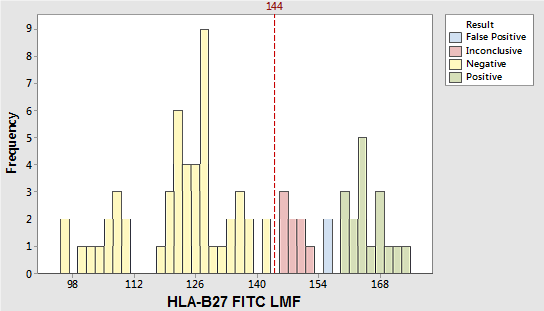
The anti–HLA-B27 antibody, clone GS145.2, used in the BD® HLA-B27 Test, has been shown to cross-react most commonly with HLA-B7.7 The LMF for some cross-reacting samples can fall on the positive side of the decision marker, thus resulting in false-positive results.
A study was performed to characterize this cross-reactivity by comparing results obtained using the HLA-B27 reagent with results obtained using either cytotoxicity or molecular testing methods. Seventy-seven specimens were stained in triplicate and acquired by each of multiple operators on two BD FACSCanto instruments. Results are shown in the figure above. All seventeen HLA-B27–positive specimens were above the decision marker plus 10 channels (LMF channel 154). Fifty HLA-B27–negative specimens were below the decision marker (LMF channel 144). Eight HLA-B27–negative specimens were within 144 and 154 LMF. Two HLA-B27–negative specimens were above 154 LMF. Based on our study and literature data8 a majority of the crossreactive specimens that fell on the positive side of the decision marker (LMF channel 144) were within 144 and 154 LMF.
This zone is referred to as the gray zone for BD FACSCalibur and BD FACSCanto systems. For the BD FACSVia system, samples falling in this zone are labeled inconclusive. Results that fall within this zone should be confirmed by an alternate method. We and other investigators have observed a small number of HLA-B27–negative specimens with LMF above this gray zone, thus resulting in false-positive results. Since the prevalence and distribution of HLA-B antigen cross-reactivity can vary9, we recommend that laboratories confirm this gray zone (inconclusive specimens) by performing their own studies.
The BD® HLA-B27 System is a qualitative two-color direct immunofluorescence method for the rapid detection of HLA-B27 antigen expression in erythrocyte-lysed whole blood (LWB) using the BD FACSVia™, BD FACSCanto™ II, BD FACSCalibur™ Flow Cytometers. Not for use in tissue typing.
-
Brochure
References
- Wang L, Wang F, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369-395. doi: 10.1111/joim.12395
- Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551-1557. doi: 10.1007/s00296-017-3726-1
- Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610-2615. doi: 10.1073/pnas.0337679100
- Chung IM, Ketharnathan S, Thiruvengadam M, Rajakumar G. Rheumatoid arthritis: the stride from research to clinical practice. Int J Mol Sci. 2016;17(6):900. doi: 10.3390/ijms17060900
- Dakwar E, Reddy J, Vale FL, Uribe JS. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E2. doi: 10.3171/FOC/2008/24/1/E2
- Chen B, Li J, He C, et al. Role of HLA-B27 in the pathogenesis of anlkylosing spondylitis. Mol Med Rep. 2017;15(4):1943-1951. doi: 10.3892/mmr.2017.6248
- Levering WHBM, Wind H, Sintnicolaas K, Hooijkaas H, Gratama JW. Flow cytometric HLAB27 screening: cross-reactivity patterns of commercially available anti-HLA-B27 monoclonal antibodies with other HLA-B antigens. Cytometry B Clin Cytom. 2003;54(1):28-38. doi: 10.1002/cyto.b.10022
- Seipp MT, Erali M, Wies RL, Wittwer C. HLA-B27
typing: evaluation of an allele-specific PCR melting assay and two flow
cytometric antigen assays. Cytometry B Clin Cytom. 2005;63:10-15.
- Johnson J, Garbutt J, Nelson KA. A monoclonal antibody specific for HLA-B27. Hum Immunol. 1985;14:134-135.
![]()
BD® HLA-B27 Kit, BD FACSCanto™II Flow Cytometer and BD FACSVia™ System are vitro diagnostic medical devices bearing a CE-mark.
BD Flow Cytometers are Class 1 Laser Products.
BD FACSCalibur™ Flow Cytometer and BD FACSVia™ System are discontinued.