Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD™ CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to BD CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD CompBead to ensure that BD CompBeads are appropriate for your specific cellular application.
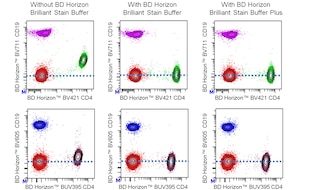
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
Product Notices
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- BD Horizon Brilliant™ Violet 750 is covered by one or more of the following US patents: 8,158,444; 8,802,450; 8,575,303; 8,455,613; 8,227,187; 8,841,072; 8,110,673.
Companion Products
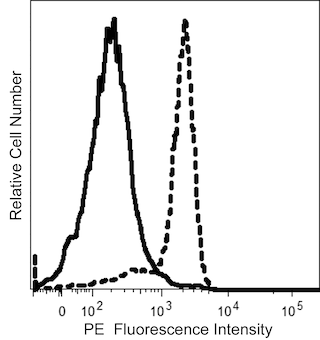




The Anti-Kappa antibody, clone TB28-2, is derived from the hybridization of P3-X63-AG8.653 mouse myeloma cells with spleen cells isolated from CB6F1/J (C57BL/6J × BALB/cJ) mice immunized with human IgG, κ myeloma protein.The Anti-Kappa antibody specifically recognizes kappa (κ) light chains of human immunoglobulins.
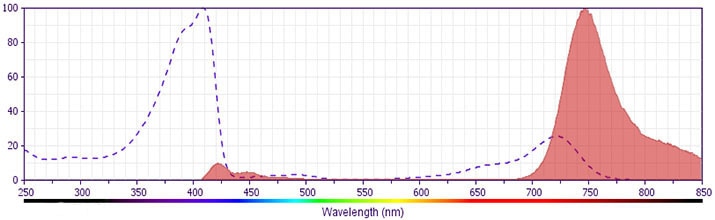
The antibody was conjugated to BD Horizon BV750 which is part of the BD Horizon Brilliant™ Violet family of dyes. This dye is a tandem fluorochrome of BD Horizon BV421 with an Ex Max of 405-nm and an acceptor dye with an Em Max at 750-nm. BD Horizon Brilliant BV750 can be excited by the violet laser (405 nm) and detected with a 750/30 nm filter with a 740 nm long pass. Due to spectral differences between labeled cells and beads, using BD™ CompBeads can result in incorrect spillover values when used with BD Horizon BV750 reagents. Therefore, the use of BD CompBeads or BD CompBeads Plus to determine spillover values for these reagents is not recommended.
Development References (13)
-
Ault KA. Flow cytometric evaluation of normal and neoplastic B cells. In: Rose NR, Friedman H, Fahey JL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clinical Laboratory Immunology. 3rd ed.. Washington, DC: American Society for Microbiology; 1986:247-253. View Reference
-
Foon KA, Todd RF. Immunologic classification of leukemia and lymphoma.. Blood. 1986; 68(1):1-31. (Methodology). View Reference
-
Harris NL, Data RE. The distribution of neoplastic and normal B-lymphoid cells in nodular lymphomas: use of an immunoperoxidase technique on frozen sections.. Hum Pathol. 1982; 13(7):610-7. (Methodology: Immunohistochemistry). View Reference
-
Kubagawa H, Gathings WE, Levitt D, Kearney JF, Cooper MD. Immunoglobulin isotype expression of normal pre-B cells as determined by immunofluorescence.. J Clin Immunol. 1982; 2(4):264-9. (Methodology: Immunofluorescence). View Reference
-
Meis JM, Osborne BM, Butler JJ. A comparative marker study of large cell lymphoma, Hodgkin's disease, and true histiocytic lymphoma in paraffin-embedded tissue.. Am J Clin Pathol. 1986; 86(5):591-9. (Methodology). View Reference
-
Picker LJ, Weiss LM, Medeiros LJ, Wood GS, Warnke RA. Immunophenotypic criteria for the diagnosis of non-Hodgkin's lymphoma.. Am J Pathol. 1987; 128(1):181-201. (Clone-specific: Immunohistochemistry). View Reference
-
Smith BR, Weinberg DS, Robert NJ, et al. Circulating monoclonal B lymphocytes in non-Hodgkin's lymphoma.. N Engl J Med. 1984; 311(23):1476-81. (Methodology: Flow cytometry). View Reference
-
Stetler-Stevenson M, Braylan RC. Flow cytometric analysis of lymphomas and lymphoproliferative disorders.. Semin Hematol. 2001; 38(2):111-23. (Methodology: Flow cytometry). View Reference
-
Stites DP, Casavant CH, McHugh TM, et al. Flow cytometric analysis of lymphocyte phenotypes in AIDS using monoclonal antibodies and simultaneous dual immunofluorescence.. Clin Immunol Immunopathol. 1986; 38(2):161-77. (Methodology: Flow cytometry). View Reference
-
Tubbs RR, Sheibani K, Weiss RA, Sebek BA, Deodhar SD. Tissue immunomicroscopic evaluation of monoclonality of B-cell lymphomas: comparison with cell suspension studies.. Am J Clin Pathol. 1981; 76(1):24-8. (Methodology: Immunohistochemistry). View Reference
-
Têtu B, Manning JT, Ordóñez NG. Comparison of monoclonal and polyclonal antibodies directed against immunoglobulin light and heavy chains in non-Hodgkin's lymphoma.. Am J Clin Pathol. 1986; 85(1):25-31. (Methodology: Immunohistochemistry). View Reference
-
Weinberg DS, Pinkus GS, Ault KA. Cytofluorometric detection of B cell clonal excess: a new approach to the diagnosis of B cell lymphoma.. Blood. 1984; 63(5):1080-7. (Methodology: Flow cytometry). View Reference
-
van Dongen JJ, Lhermitte L, Böttcher S, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012; 26(9):1908-1975. (Methodology: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.