Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




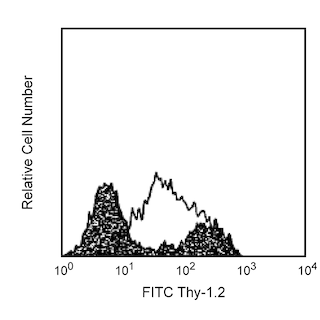
Flow cytometric analysis of CD44 expression on mouse bone-marrow cells. BALB/c mouse bone-marrow cells were preincubated with Purified Rat Anti-Mouse CD16/CD32 antibody (Mouse BD Fc Block™) (Cat. No. 553141/553142). The cells were then stained with either BD Horizon™ BV480 Rat IgG2b, κ Isotype Control (Cat. No. 565649; dashed line histogram) or BD Horizon BV480 Rat Anti-Mouse CD44 antibody (Cat. No. 566116/566200; solid line histogram). The fluorescence histogram showing CD44 expression (or Ig Isotype control staining) was derived from gated events with the forward and side light-scatter characteristics of viable bone marrow cells. Flow cytometric analysis was performed using a BD LSRFortessa™ Cell Analyzer System.


BD Horizon™ BV480 Rat Anti-Mouse CD44

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant™ Stain Buffer should be used anytime BD Horizon Brilliant™ dyes are used in a multicolor flow cytometry panel. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. When BD Horizon Brilliant Stain Buffer is used in in the multicolor panel, it should also be used in the corresponding compensation controls for all dyes to achieve the most accurate compensation. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- BD Horizon Brilliant Violet 480 is covered by one or more of the following US patents: 8,575,303; 8,354,239.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products





The IM7 antibody specifically recognizes an epitope on both alloantigens and all isoforms of the CD44 glycoprotein (Pgp-1, Ly-24). The standard form of CD44, lacking variable exons and referred to as CD44H or CD44s, is widely expressed on hematopoietic and non-hematopoietic cells. CD44 isoforms encoded by variable exons are expressed on epithelial cells, but only at low levels on most leukocytes. Mice with the Ly-24.1 alloantigen (e.g., BALB/c, CBA/J, DBA/1, DBA/2) have relatively large subsets of CD44H+ T lymphocytes, while Ly-24.2 strains (e.g., A, AKR, CBA/N, C3H/He, C57BL, C57BR, C57L, C58, NZB, SJL, SWR, 129) have fewer CD44H+ T cells. CD44 is a cell adhesion receptor, and its principal ligand, hyaluronate, is a common component of extracellular matrices. Differential glycosylation of CD44 influences its binding to hyaluronate. Additional ligands include the cell surface form of CD74 and the cytokine osteopontin (Eta-1). Bone marrow- and thymus-derived progenitor cells capable of repopulating the thymus express CD44. In the periphery, the level of CD44 expression increases upon activation of B lymphocytes, CD4+ T cells, and CD8+ T cells; memory cells can be recognized by their CD44[hi] phenotype. The IM7 mAb inhibits established collagen-induced arthritis in DBA/1 mice. Moreover, it prevents CNS inflammation and clinical symptoms of experimental autoimmune encephalomyelitis. In contrast, the same antibody exacerbates experimental autoimmune thyroiditis in CBA/J mice. The IM7 mAb recognizes a different epitope from that recognized by mAb KM114, and the antibody pair can be used in ELISA to detect soluble CD44. It has been observed that IM7 antibody crossreacts with human, dog, cat, horse, cow, and pig leukocytes. Anti-human CD44, clone G44-26, and IM7 antibody compete for binding to human peripheral blood lymphocytes.
Development References (18)
-
Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci U S A. 1999; 96(12):6896-6901. (Clone-specific: Blocking). View Reference
-
Budd RC, Cerottini JC, Horvath C, et al. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol. 1987; 138(10):3120-3129. (Clone-specific: Flow cytometry, Fluorescence activated cell sorting, Immunoprecipitation). View Reference
-
Camp RL, Scheynius A, Johansson C, Pure E. CD44 is necessary for optimal contact allergic responses but is not required for normal leukocyte extravasation. J Exp Med. 1993; 178(2):497-507. (Clone-specific: Induction, Inhibition, Radioimmunoassay). View Reference
-
Ernst DN, Weigle WO, Noonan DJ, McQuitty DN, Hobbs MV. The age-associated increase in IFN-γ synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J Immunol. 1993; 151(2):575-587. (Clone-specific: Flow cytometry). View Reference
-
Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993; 150(10):4244-4252. (Clone-specific: Flow cytometry, Fluorescence activated cell sorting). View Reference
-
Hathcock KS, Hirano H, Murakami S, Hodes RJ. CD44 expression on activated B cells. Differential capacity for CD44-dependent binding to hyaluronic acid. J Immunol. 1993; 151(12):6712-6722. (Clone-specific: Flow cytometry, Immunoprecipitation). View Reference
-
Hyman R, Lesley J, Schulte R, Trotter J. Progenitor cells in the thymus: most thymus-homing progenitor cells in the adult mouse thymus bear Pgp-1 glycoprotein but not interleukin-2 receptor on their cell surface. Cell Immunol. 1986; 101(2):320-327. (Clone-specific: Flow cytometry). View Reference
-
Katoh S, McCarthy JB, Kincade PW. Characterization of soluble CD44 in the circulation of mice. Levels are affected by immune activity and tumor growth. J Immunol. 1994; 153(8):3440-3449. (Clone-specific: ELISA). View Reference
-
Katoh S, Zheng Z, Oritani K, Shimozato T, Kincade PW. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med. 1995; 182(2):419-429. (Clone-specific: Blocking). View Reference
-
Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993; 54:271-335. (Biology). View Reference
-
Lesley J, Trowbridge IS. Genetic characterization of a polymorphic murine cell-surface glycoprotein. Immunogenetics. 1982; 15(3):313-320. (Clone-specific: Immunoaffinity chromatography, Immunoprecipitation, Radioimmunoassay). View Reference
-
Lynch F, Ceredig R. Mouse strain variation in Ly-24 (Pgp-1) expression by peripheral T cells and thymocytes: implications for T cell differentiation. Eur J Immunol. 1989; 19(2):223-229. (Clone-specific: Flow cytometry). View Reference
-
MacDonald HR, Budd RC, Cerottini JC. Pgp-1 (Ly 24) as a marker of murine memory T lymphocytes. Curr Top Microbiol Immunol. 1990; 159:97-109. (Biology). View Reference
-
Matsumoto G, Nghiem MP, Nozaki N, Schmits R, Penninger JM. Cooperation between CD44 and LFA-1/CD11a adhesion receptors in lymphokine-activated killer cell cytotoxicity. J Immunol. 1998; 160(12):5781-5789. (Clone-specific: Flow cytometry). View Reference
-
Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997; 71:241-319. (Biology). View Reference
-
Nedvetzki S, Walmsley M, Alpert E, Williams RO, Feldmann M, Naor D. CD44 involvement in experimental collagen-induced arthritis (CIA). J Autoimmun. 1999; 13(1):39-47. (Clone-specific: Blocking). View Reference
-
Trowbridge IS, Lesley J, Schulte R, Hyman R, Trotter J. Biochemical characterization and cellular distribution of a polymorphic, murine cell-surface glycoprotein expressed on lymphoid tissues. Immunogenetics. 1982; 15:299-312. (Immunogen: Cytotoxicity, Depletion, Flow cytometry, Functional assay, Immunoprecipitation). View Reference
-
Vremec D, Zorbas M, Scollay R, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992; 176(1):47-58. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.