BD Pharmingen™ 647 EdU Click Proliferation Kit
(RUO)





Description
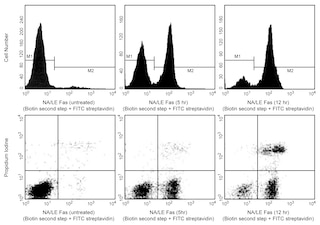
The BD Pharmingen™ 647 EdU Click Proliferation Kit provides a tool for analyzing DNA synthesis in cycling cells. In this method, the thymidine analog EdU (5-ethynyl-2´-deoxyuridine) is incorporated into newly synthesized DNA by cells progressing through the S (DNA synthesis) phase of the cell cycle. EdU can then be detected in fixed and permeabilized cells with a fluorophore-labeled azide. The reaction of the fluorophore-labeled azide and EdU occurs via a copper-catalyzed click reaction between the azide moiety of the fluorophore and the alkyne moiety of EdU. When co-stained with a DNA dye such as 7-AAD, PI, or DAPI, cell populations may be segmented by flow cytometry into the G0/G1-phases (2N DNA content, EdU-negative), S-phase (2N-4N DNA content, EdU-positive), or G2/M-phases (4N DNA content, EdU-negative).
The BD Pharmingen™ 647 EdU Click Proliferation Kit contains Eterneon™ Red 645 Azide, which is excited by the red laser and has an excitation maximum of 643 nm and an emission maximum of 662 nm.
Please note that this kit is provided as Part 1 of 2 (Components A, B, and C) to be stored dry and protected from light at -20°C, and Part 2 of 2 (Components D, E, F, and G) to be stored dry at 2 - 8°C or room temperature. See "Kit Contents" immediately below for more information.
Kit Contents
Component ID Component Description Amount Long Term Storage Conditions
A EdU (5-ethynyl-2´-deoxyuridine) 10 mg -20°C, dry
B Eterneon™ Red 645 azide (10 mM) 130 μL -20°C, dry, dark
C Buffer Additive (10×) 400 mg -20°C, dry
D Saponin-based Permeabilization and Wash Reagent (10×) 50 mL 2 - 8°C
E Fixative Solution (4% paraformaldehyde-based) 5 mL 2 - 8°C
F Catalyst Solution 2 mL RT, dry
G DMSO 5 mL RT, dry
Please note that Hazard Warnings for components listed above are found on Page 5 of this document.
Recommended Assay Procedures
A. Materials Required but Not Provided
• Cells of interest
• FACS tubes or imaging plates/slides
• Buffered saline solution, such as PBS, DPBS, or TBS
• BD Pharmingen™ Stain Buffer (BSA) (Cat. No. 554657) or BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656)
• Deionized water
• Fluorescent antibodies or dyes for immunophenotypic or functional analysis (optional)
• DNA content dye (optional)
B. Cytometry Requirements
Red laser-equipped cytometers (BD FACSCanto™ II, BD LSRFortessa™, BD LSR™ II, and BD Accuri™ C6 Flow Cytometer) can be used. Eterneon™ Red 645 can be read out of filters commonly used for APC or Alexa Fluor® 647 (eg, 670/30 bandpass filter). Fluorescence compensation is best achieved using a sample of the cells of interest.
EdU incorporation is best analyzed with logarithmic amplification, while total DNA content is best analyzed with linear amplification. If DNA content is to be assessed, samples should be acquired on a low flow rate to generate the best resolution of 2N and 4N populations.
C. Preparation of Stock Solutions
1. Allow all vials to warm to room temperature before opening.
2. Component A: For the preparation of a 10 mM stock solution of EdU, add 4 mL DMSO or 1× PBS to EdU and mix until completely dissolved. After use, store any remaining solution at -20°C. When stored as directed, this stock solution is stable for up to one year. We recommend preparing aliquots to avoid repeated freeze-thaw cycles.
3. Component B: For Eterneon™ Red 645 azide, we recommend preparing aliquots to avoid repeated freeze-thaw cycles. Aliquots should be stored dry and protected from light at -20°C.
4. Component C: For the preparation of a 10× stock solution of the buffer additive, add 4 mL deionized water to the buffer additive and mix until completely dissolved. After use, store any remaining solution at -20°C. When stored as directed, this stock solution is stable for up to 6 months. If the solution starts to develop a brown color, it has degraded and should be discarded. We recommend preparing aliquots to avoid repeated freeze-thaw cycles.
5. Component D: For the preparation of 500 mL of 1× saponin-based permeabilization buffer and wash reagent, add 50 mL Component D to 450 mL 1% BSA in 1× PBS. After use, store any remaining solution at 2 - 8°C.
D. Recommended Staining Procedure - Flow Cytometry
Labeling of Cells with EdU
1. Add desired amount of EdU to cells in culture medium. We have found a final concentration of 10 μM EdU to be sufficient for labeling of human and mouse cell lines and primary cell populations.
a. During addition of EdU to cells in culture, avoid disturbing the cells in any way (eg, centrifugation steps or temperature changes) that may disrupt the normal cell cycling patterns. The cell culture density should not exceed 2 × 10^6 cells/mL.
2. Incubate the treated cells for the desired length of time. Different cell types may require different incubation periods for optimal labeling with EdU. As a starting point, we recommend 10 μM EdU for 1 hour.
3. Harvest cells, pellet by centrifugation, and remove EdU-containing media.
4. Dislodge the cell pellet, resuspend cells in BD Pharmingen™ Stain Buffer (BSA) (Cat. No. 554657) or BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) at 1 × 10^7 cells/mL, and aliquot 1 × 10^6 cells per 12 × 75-mm FACS tube.
5. If you do not wish to stain for surface antigens, add 1 mL BD Pharmingen™ Stain Buffer, pellet cells by centrifugation, remove the supernatant, and proceed to step D9.
Staining of Cell Surface Antigens (Optional)
6. Add fluorescent antibodies specific for cell surface markers to tubes and vortex gently to mix.
a. Because the click reaction cocktail contains copper and copper is known to quench the fluorescence of R-PE, antibody conjugates containing R-PE or R-PE tandems should be stained after the click reaction (Staining of Intracellular and/or Surface Antigens, steps D19-22).
7. Incubate samples for 30 minutes at room temperature or on ice.
8. Wash samples by adding 1 mL BD Pharmingen™ Stain Buffer, pelleting by centrifugation, and removing the supernatant.
Fixing and Permeabilizing the Cells
9. Dislodge the cell pellet and resuspend cells in 100 μL Fixative Solution (Component E) per tube.
10. Incubate the cells for 15 minutes at room temperature.
11. Wash samples by adding 1 mL BD Pharmingen™ Stain Buffer, pelleting by centrifugation, and removing the supernatant.
12. Dislodge the cell pellet, resuspend the cells in 100 μL of 1× Saponin-Based Permeabilization Buffer (prepared in step C5), and vortex gently to mix.
Detection of EdU Incorporation
13. Prepare the assay cocktail as described in Table I after section G, adding reagents in the same order indicated in the table. If the ingredients are not added in the order listed, the reaction may not proceed optimally or might fail. Once the assay cocktail is prepared, use it within the next 15 minutes.
a. Please note that in some cases, titration of the dye azide (Component B) can increase resolution of EdU positive and negative populations. In this case, dilute an aliquot of Component B with an appropriate amount of DMSO and add 1 μL of the diluted dye azide in place of the concentrated stock.
14. Add 500 μL of the assay cocktail to each sample and vortex gently to mix.
15. Incubate the samples for 30 minutes at room temperature protected from light.
16. Wash samples by adding 2 mL 1× Saponin-Based Permeabilization Buffer (prepared in step C5), pelleting by centrifugation, and removing the supernatant.
17. Repeat step D16.
18. If you do not wish to stain for intracellular antigens or DNA content, dislodge the cell pellet and resuspend cells in 500 μL 1× PBS or equivalent, and proceed to analysis on the flow cytometer.
Staining of Intracellular and/or Surface Antigens (Optional)
19. Dislodge the cell pellet and resuspend cells in 100 μL BD Pharmingen™ Stain Buffer.
20. Add fluorescent antibodies specific for intracellular and/or surface markers to tubes and vortex gently to mix.
a. Because the click reaction cocktail contains copper and copper is known to quench the fluorescence of R-PE, antibody conjugates containing R-PE or R-PE tandems should be stained at this point in the procedure.
21. Incubate samples for 30 minutes at room temperature or on ice.
22. Wash samples by adding 1 mL BD Pharmingen™ Stain Buffer, pelleting by centrifugation, and removing the supernatant.
Staining for Total DNA Content (Optional)
23. Dislodge the cell pellet and resuspend in 500 μL 1× PBS containing an appropriate amount of DNA dye (eg, DAPI, PI, or 7-AAD) for your cell type of interest. Vortex gently to mix.
24. Proceed to analysis by flow cytometry.
E. Recommended Staining Procedure - Bioimaging
This assay was optimized for 96-well plate imaging. Reagent volumes may need to be optimized for other imaging geometries.
Labeling of Cells with EdU
1. Culture desired cell type in well-plates.
2. Add desired amount of EdU to cells in culture medium, or replace culture medium with fresh, pre-warmed medium containing desired amount of EdU. We have found a final concentration of 10 μM EdU to be sufficient for labeling of human and mouse cell lines and primary cell populations.
a. During addition of EdU to cells in culture, avoid disturbing the cells in any way (eg, centrifugation steps or temperature changes) that may disrupt the normal cell cycling patterns. The cell culture density should not exceed 70% confluence.
3. Incubate the treated cells for the desired length of time. Different cell types may require different incubation periods for optimal labeling with EdU.
4. Remove EdU-containing media, wash cells once in BD Pharmingen™ Stain Buffer, and remove wash buffer. If you do not wish to stain for surface antigens, proceed to step E8.
Staining of Cell Surface Antigens (Optional)
5. Prepare fluorescent antibodies specific for cell surface markers in BD Pharmingen™ Stain Buffer, and add to samples. Stain volume should be large enough to cover the well entirely (eg, at least 50 μL for a 96-well).
6. Incubate samples for 60 minutes at room temperature.
7. Wash samples twice with BD Pharmingen™ Stain Buffer, and remove wash buffer.
Fixing and Permeabilizing the Cells
8. Add Fixative Solution (Component E) to each sample. Fixative Solution volume should be large enough to cover the well entirely (eg, at least 50 μL for a 96-well).
9. Incubate samples for 15 minutes at room temperature.
10. Wash samples twice with BD Pharmingen™ Stain Buffer, and remove wash buffer.
11. Add 1× Saponin-Based Permeabilization Buffer (prepared in step C5) to each sample. Permeabilization Buffer volume should be large enough to cover the well entirely (eg, at least 50 μL for a 96-well).
12. Incubate samples for 15 minutes at room temperature.
Detection of EdU Incorporation
13. Make up a working solution of the dye azide (Component B) by diluting 1:5 with DMSO (eg, dilute 1 μL dye azide with 4 μL DMSO). Vortex well to mix.
14. Prepare the assay cocktail as described in Table II after section G, adding reagents in the same order indicated in the table. If the ingredients are not added in the order listed, the reaction may not proceed optimally or might fail. Once the assay cocktail is prepared, use it within the next 15 minutes.
a. Please note that in some cases, titration of the dye azide (Component B) can increase resolution of EdU positive and negative populations. In this case, dilute the working solution of dye azide prepared in step E13 with an appropriate amount of DMSO and add 1 μL of the diluted working solution in place of the concentrated working solution.
15. Add 100 μL of the assay cocktail to each sample, on top of the 50 μL 1× Saponin-Based Permeabilization Buffer added in step E11. If necessary, more cocktail may be used per sample, but all components should be kept in the same ratios and added in the same order in order for the reaction to proceed optimally.
16. Incubate the samples for 30 minutes at room temperature, protected from light.
17. Wash samples twice with 1× Saponin-Based Permeabilization Buffer (prepared in step C5), 5 minutes per wash, and remove wash buffer.
18. If you do not wish to stain for intracellular antigens or DNA content, add PBS to each sample and proceed to imaging. PBS volume should be enough to completely cover the wells entirely (eg, at least 50 μL for a 96-well).
Staining of Intracellular and/or Surface Antigens (Optional)
19. Prepare fluorescent antibodies specific for intracellular and/or surface markers in BD Pharmingen™ Stain Buffer, and add to samples. Stain volume should be large enough to cover the well entirely (eg, at least 50 μL for a 96-well).
20. Incubate samples for 60 minutes at room temperature.
21. Wash samples twice with BD Pharmingen™ Stain Buffer, and remove wash buffer.
Staining for Total DNA Content (Optional)
22. Add 100 μL 1× PBS containing an appropriate amount of DNA dye for your cell type of interest.
23. Proceed to analysis by bioimaging.
F. Notes
1. The click reaction cocktail contains copper, which is known to quench the fluorescence of R-PE. Antibody conjugates containing PE or R-PE should be stained after the click reaction.
2. The copper present in the click reaction cocktail may affect binding of anti-GFP antibodies to their epitopes. We recommend staining with anti-GFP antibodies before the click reaction.
3. Organic dyes (eg, Alexa Fluor® dyes), PerCP-Cy™5.5, APC, Brilliant™ Violet, and Brilliant™ Ultraviolet dyes have been found to be compatible with the click reaction.
4. Large shifts in autofluorescence can occur from exposure to the click cocktail. We recommend that all controls, including unstained and single stained controls, also be treated with the click cocktail with or without the dye azide as appropriate so that control and test samples exhibit the same autofluorescent properties.
G. Hazards identification
Warning: Component B, Eterneon™ Red 645 azide (10 mM), Material Number 51-9012111, contains 99.4% DMSO.
Hazard statements
Combustible liquid.
Precautionary statements
Keep away from flames and hot surfaces. - No smoking.
Wear protective gloves / eye protection.
Wear protective clothing.
In case of fire: Use for extinction: CO2, powder or water spray.
Store in a well-ventilated place. Keep cool.
Danger: Component E, Fixative Solution, Material Number 51-9012105, contains 4% formaldehyde.
Hazard statements
Harmful if inhaled.
Causes skin irritation.
Causes serious eye damage.
May cause an allergic skin reaction.
Suspected of causing genetic defects.
May cause cancer. Route of exposure: Inhalation.
May cause respiratory irritation.
Precautionary statements
Do not breathe mist/vapours/spray.
Wear protective clothing / eye protection.
Wear protective gloves.
If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue
rinsing.
If skin irritation or rash occurs: Get medical advice/attention.
Dispose of contents/container in accordance with local/regional/national/international regulations.
Component F, Catalyst solution, Material Number 51-9012106, contains 1.6% copper sulfate.
Hazard statements
Harmful to aquatic life with long lasting effects.
· Precautionary statements
Avoid release to the environment.
Dispose of contents/container in accordance with local/regional/national/international regulations.
Warning: Component G, Material Number 51-9012107, contains DMSO.
Hazard statements
Combustible liquid.
Precautionary statements
Keep away from flames and hot surfaces. - No smoking.
Wear protective gloves / eye protection.
Wear protective clothing.
In case of fire: Use for extinction: CO2, powder or water spray.
Store in a well-ventilated place. Keep cool.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Eterneon™ is a trademark of baseclick GmbH
- Limited Use Label License — Research Use Only: The purchase of this product conveys to the purchaser the limited, non-transferable right to use the purchased amount of the product only to perform research, and not for any clinical, diagnostic, vaccine, protective, prophylactic, preventive or therapeutic use in humans, animals, or plants.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- This product is provided under an intellectual property license from Life Technologies Corporation. The transfer of this product is conditioned on the buyer using the purchased product solely in research conducted by the buyer, excluding contract research or any fee for service research, and the buyer must not sell or otherwise transfer this product or its components for (a) diagnostic, therapeutic or prophylactic purposes; (b) testing, analysis or screening services, or information in return for compensation on a per-test basis; (c) manufacturing or quality assurance or quality control, or (d) resale, whether or not resold for use in research. For information on purchasing a license to this product for purposes other than as described above, contact Life Technologies Corporation, 5791 Van Allen Way, Carlsbad, CA 92008 USA or outlicensing@lifetech.com.
Companion Products




Development References (6)
-
Buck SD, Bradford J, Gee KR, Agnew BJ, Clarke ST, Salic A. Detection of S-phase cell cycle progression using 5-ethynyl-2’-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2’-deoxyuridine antibodies. Biotechniques. 2008; 44(7):927-929. (Methodology). View Reference
-
Cavanagh BL, Walker T, Norazit A, Meedeniya AC. Thymidine analogues for tracking DNA synthesis. Molecules. 2011; 16(9):7980-7993. (Methodology). View Reference
-
Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001; 40(11):2004-2021. (Methodology). View Reference
-
Kotogany E, Dudits D, Horvath GV, Ayaydin F. A rapid and robust assay for detection of S-phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods. 6(1)(Methodology). View Reference
-
Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008; 105(7):2415-2420. (Methodology). View Reference
-
Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by Copper(I)-Catalyzed Azide-Alkyne [3 + 2] Cycloaddition. J Am Chem Soc. 2003; 125(11):3192-3193. (Methodology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.