-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- France (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Alexa Fluor® 555 Mouse anti-Nestin
Clone 25/NESTIN (RUO)
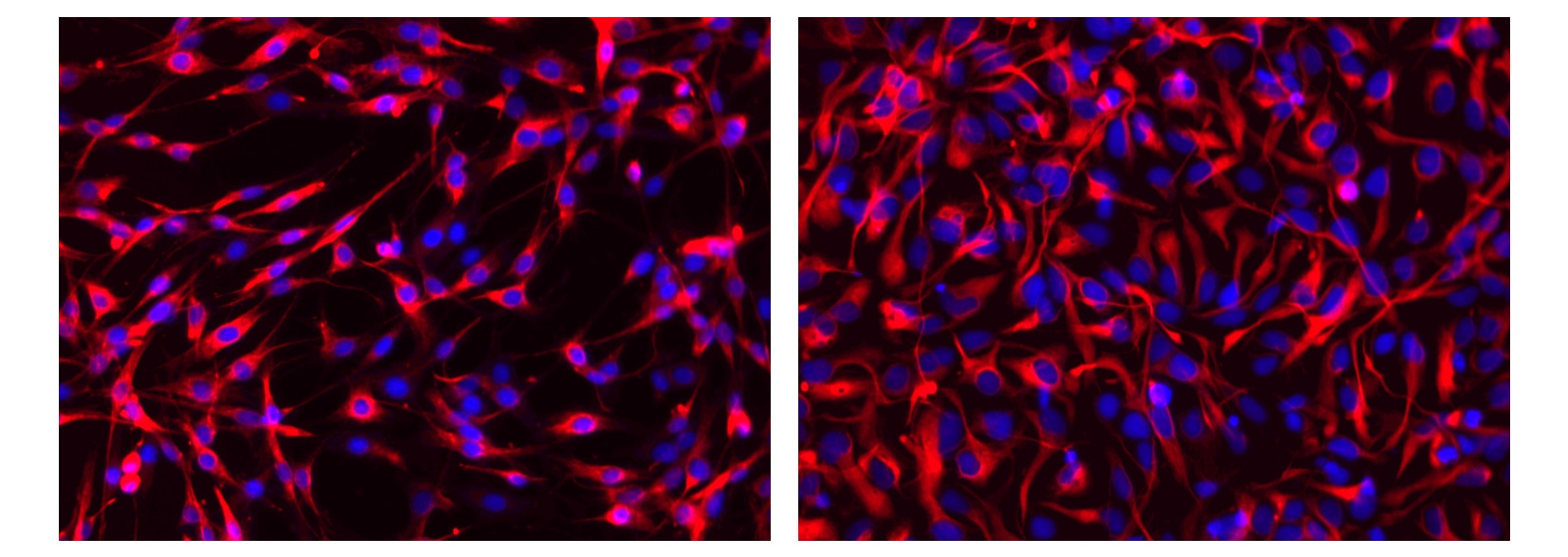
Immunofluorescent staining of rat and human cells, respectively. C6 glioma cells (ATCC Cat. No. CCL-107, left image) and neural stem cells derived from H9 embryonic stem cells (WiCell, Madison, WI, right image) were cultured in a 96-well imaging plate (Cat. No. 353219). Cells were fixed, permeabilized with cold methanol, and stained with Alexa Fluor® 555 Mouse anti-Nestin (pseudo colored red) according to the Recommended Assay Procedure. Cell nuclei were counterstained with Hoechst 33342 (pseudo colored blue). The images were captured on a BD Pathway™ 435 High-Content Bioimager System using a 20X objective and merged using BD AttoVision™ software. The staining also worked with the Saponin and the Triton™ X-100 Perm/Wash protocols (see Recommended Assay Procedure; Bioimaging protocol link).


Immunofluorescent staining of rat and human cells, respectively. C6 glioma cells (ATCC Cat. No. CCL-107, left image) and neural stem cells derived from H9 embryonic stem cells (WiCell, Madison, WI, right image) were cultured in a 96-well imaging plate (Cat. No. 353219). Cells were fixed, permeabilized with cold methanol, and stained with Alexa Fluor® 555 Mouse anti-Nestin (pseudo colored red) according to the Recommended Assay Procedure. Cell nuclei were counterstained with Hoechst 33342 (pseudo colored blue). The images were captured on a BD Pathway™ 435 High-Content Bioimager System using a 20X objective and merged using BD AttoVision™ software. The staining also worked with the Saponin and the Triton™ X-100 Perm/Wash protocols (see Recommended Assay Procedure; Bioimaging protocol link).

Immunofluorescent staining of rat and human cells, respectively. C6 glioma cells (ATCC Cat. No. CCL-107, left image) and neural stem cells derived from H9 embryonic stem cells (WiCell, Madison, WI, right image) were cultured in a 96-well imaging plate (Cat. No. 353219). Cells were fixed, permeabilized with cold methanol, and stained with Alexa Fluor® 555 Mouse anti-Nestin (pseudo colored red) according to the Recommended Assay Procedure. Cell nuclei were counterstained with Hoechst 33342 (pseudo colored blue). The images were captured on a BD Pathway™ 435 High-Content Bioimager System using a 20X objective and merged using BD AttoVision™ software. The staining also worked with the Saponin and the Triton™ X-100 Perm/Wash protocols (see Recommended Assay Procedure; Bioimaging protocol link).


BD Pharmingen™ Alexa Fluor® 555 Mouse anti-Nestin

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For more information, please refer to: http://www.bdbiosciences.com/pharmingen/protocols/Bioimaging_Certified.shtml or htpp://www.bdbiosciences.com/bioimaging/reagents.
Recommended Protocol for Bioimaging:
1. Seed the cells in appropriate culture medium at an appropriate cell density in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219), and
culture overnight to 48 hours.
2. Remove the culture medium from the wells, and wash (one to two times) with 100 μl of 1× PBS.
3. Fix the cells by adding 100 µl of fresh 3.7% Formaldehyde in PBS or BD Cytofix™ fixation buffer (Cat. No. 554655) to each well and incubating for 10 minutes at room temperature (RT).
4. Remove the fixative from the wells, and wash the wells (one to two times) with 100 μl of 1× PBS.
5. Permeabilize the cells using either cold methanol (a), Triton™ X-100 (b), or Saponin (c):
a. Add 100 µl of -20°C 90% methanol or -20°C BD Phosflow™ Perm Buffer III (Cat. No. 558050) to each well and incubate for 5 minutes at RT.
b. Add 100 µl of 0.1% Triton™ X-100 to each well and incubate for 5 minutes at RT.
c. Add 100 µl of 1× Perm/Wash buffer (Cat. No. 554723) to each well and incubate for 15 to 30 minutes at RT. Continue to use 1× Perm/Wash buffer for all subsequent wash and dilutions steps.
6. Remove the permeabilization buffer from the wells, and wash one to two times with 100 μl of appropriate buffer (either 1× PBS or 1× Perm/Wash buffer, see step 5.c.).
7. Optional blocking step: Remove the wash buffers, and block the cells by adding 100 µl of blocking buffer BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) or 3% FBS in appropriate dilution buffer to each well and incubating for 15 to 30 minutes at RT.
8. Dilute the antibody to its optimal working concentration in appropriate dilution buffer. Titrate purified (unconjugated) antibodies and second-step reagents to determine the optimal concentration. If using a Bioimaging Certified antibody conjugate, dilute it 1:10.
9. Add 50 µl of diluted antibody per well and incubate for 60 minutes at RT. Incubate in the dark if using fluorescently labeled antibodies.
10. Remove the antibody, and wash the wells three times with 100 μl of wash buffer. An optional detergent wash (100 μl of 0.05% Tween in 1× PBS) can be included prior to the regular wash steps.
11. If the antibody being used is fluorescently labeled, then move to step 12. Otherwise, if using a purified unlabeled antibody, repeat steps 8 to 10 with a fluorescently labeled second-step reagent to detect the purified antibody.
12. After the final wash, counterstain the nuclei by adding 100 μl of a 2 μg/ml solution of Hoechst 33342 (eg, Sigma-Aldrich Cat. No. B2261) in 1× PBS to each well at least 15 minutes before imaging.
13. View and analyze the cells on an appropriate imaging instrument. Recommended filters for the BD Pathway™ instruments are:
Instrument Excitation Emission Dichroic
BD Pathway 855 548/20 570LP Fura/FITC
BD Pathway 435 543/22 593/40 FF562
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- This reagent has been pre-diluted for use at the recommended Volume per Test when following the Recommended Assay Procedure. A Test is typically ~10,000 cells cultured in a well of a 96-well imaging plate.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- The Alexa Fluor®, Pacific Blue™, and Cascade Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, excluding use in combination with microarrays, or as analyte specific reagents. The Alexa Fluor® dyes (except for Alexa Fluor® 430), Pacific Blue™ dye, and Cascade Blue® dye are covered by pending and issued patents.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Triton is a trademark of the Dow Chemical Company.
Data Sheets
Companion Products



The cytoskeleton consists primarily of core structural proteins that include microfilaments, microtubules, and intermediate filaments (IFs). IFs contain more than 50 distinct proteins that are organized into six different subtypes: Type I/II keratins expressed in epithelia, type III vimentin/desmin, type IV neurofilament proteins, type V nuclear lamins, and type VI nestin expressed primarily in embryonic cells. Nestin has a conserved core region (amino acids 7 to 314), which contains an α helical domain that is involved in coiled-coil assembly of IFs. The C-terminal region of nestin is similar to type IV IFs, since it contains highly charged amino acids, many glutamate residues, and an 11 amino acid repeat motif. Nestin is expressed in the cerebrum during embryonic development, in the cerebellum during early postnatal development, and in dermatomal cells and myoblasts during myogenesis. In vitro, nestin forms homodimers and homotetramers, but not IFs, and can co-assemble with type III vimentin and type IV internexin proteins. Thus, nestin is a core IF protein that is essential for proper cytoskeletal formation during neurogenesis and myogenesis.
Development References (5)
-
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002; 99(18):11946-11950. (Clone-specific: Immunofluorescence). View Reference
-
Kachinsky AM, Dominov JA, Miller JB. Myogenesis and the intermediate filament protein, nestin. Dev Biol. 1994; 165(1):216-228. (Biology). View Reference
-
Kernie SG, Erwin TM, Parada LF. Brain remodeling due to neuronal and astrocytic proliferation after controlled cortical injury in mice. J Neurosci Res. 2001; 66(3):317-326. (Clone-specific: Immunofluorescence). View Reference
-
Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990; 60(4):585-595. (Biology). View Reference
-
Steinert PM, Chou YH, Prahlad V, et al. A high molecular weight intermediate filament-associated protein in BHK-21 cells is nestin, a type VI intermediate filament protein. Limited co-assembly in vitro to form heteropolymers with type III vimentin and type IV alpha-internexin. J Biol Chem. 1999; 274(14):9881-9890. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.