Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

Expression of IL-3 by stimulated CD4+ Balb/c spleen cells. Purified splenic CD4+ cells from 6 month old BALB/c mice were stimulated with plate-bound anti-CD3 (25 µg/ml final concentration; 145-2C11, Cat. No. 553057) and soluble anti-mouse CD28 (2 µg /ml final concentration; clone 37.51, Cat. No. 553294) for 2 days in culture together with recombinant mouse IL-2 (10 ng/ml final concentration; Cat. No. 550069) and recombinant mouse IL-4 (0.5 ng/ml final concentration; Cat. No. 550067), followed by a 3 day incubation with only recombinant mouse IL-2 and recombinant mouse IL-4. This was followed by a 5 hour stimulation with plate-bound anti-CD3 (25 µg/ml final concentration) and anti-mouse CD28 (2 µg/ml final concentration) in the presence of GolgiStop™ (2 µM final concentration; Cat. No. 554724). The cells were harvested, stained with 0.06 µg of FITC-conjugated rat anti-mouse CD4 (FITC-RM4-5, Cat. No. 553047), fixed, permeabilized, and subsequently stained with 0.12 µg of PE-conjugated rat anti-mouse IL-3 antibody (PE-MP2-8F8, Cat. No. 554383) by using the BD Pharmingen staining protocol (left panel). To demonstrate specificity of staining, the binding by PE-MP2-8F8 was blocked by each of the following: 1) preincubation of the conjugated antibody with molar excess of recombinant mouse IL-3 (0.12 µg, Cat. No. 554579; center panel) and by 2) preincubation of the fixed/permeabilized cells with excess unlabeled MP2-8F8 mouse antibody (3 µg; Cat. No. 554380; right panel) prior to staining with the PE-MP2-8F8. The quadrant markers for the bivariate dot plots were set based on the autofluorescence controls and verified using the recombinant cytokine blocking and unlabeled antibody blocking specificity controls.
.png)

BD Pharmingen™ PE Rat Anti-Mouse IL-3
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Immunofluorescent Staining and Flow Cytometric Analysis: The MP2-8F8 antibody is useful for immunofluorescent staining and flow cytometric analysis to identify and enumerate IL-3 producing cells within mixed cell populations. PE-conjugated MP2-8F8 antibody (Cat. No. 554383) is especially suitable for these studies (see Figure, left panel). For optimal immunofluorescent staining with flow cytometric analysis, this anti-cytokine antibody should be titrated (≤ 0.25 µg mAb/million cells). For specific methodology, please visit the protocols section or chapter on intracellular staining in the Immune Function Handbook, both of which are posted on our web site, www.bdbiosciences.com.
A useful control for demonstrating specificity of staining is either of the following: 1) pre-block the conjugated MP2-8F8 antibody with a molar excess of ligand (e.g., recombinant mouse IL-3; Cat No. 554579) prior to staining, or 2) pre-block the fixed/permeabilized cells with unlabeled MP2-8F8 antibody (Cat. No. 554380, 554381) prior to staining. The staining technique and use of blocking controls are described in detail by C. Prussin and D. Metcalfe. A suitable rat IgG1 isotype control for assessing the level of background staining on paraformaldehyde-fixed/saponin-permeabilized mouse or human cells is PE-R3-34 (Cat. No. 554685); use at comparable concentrations to antibody of interest (e.g., ≤ 0.25 µg mAb/1 million cells).
Neutralization: The NA/LE™ MP2-8F8 antibody (Cat. No. 554379) is useful for neutralization of mouse IL-3 bioactivity.
ELISA Capture: The purified MP2-8F8 antibody (Cat. No. 554380) is useful as a capture antibody for a sandwich ELISA for measuring mouse IL-3 protein levels. For specific methodology, please visit the protocols section or chapter on ELISA in the Immune Function Handbook, both of which are posted on our web site, www.bdbiosciences.com. For testing mouse IL-3 in complex biological fluids such as serum or plasma, our mouse IL-3 specific OptEIA™ sandwich ELISA set is recommended (Cat. No. 555228).
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
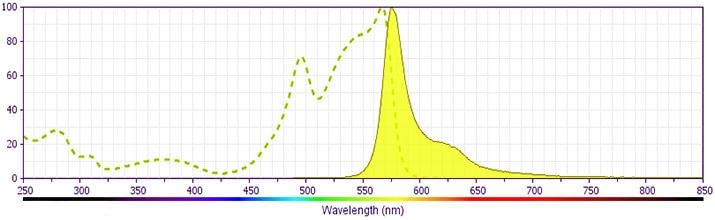
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
The MP2-8F8 antibody reacts with mouse interleukin-3 (IL-3). The immunogen used to generate the MP2-8F8 hybridoma was COS-expressed recombinant mouse IL-3.
Development References (6)
-
Abrams J. Immunoenzymetric assay of mouse and human cytokines using NIP-labeled anti-cytokine antibodies. Curr Protoc Immunol. 2001; 1:6.20-6.21. (Clone-specific: ELISA). View Reference
-
Abrams JS, Pearce MK.. Development of rat anti-mouse interleukin 3 monoclonal antibodies which neutralize bioactivity in vitro. J Immunol. 1988; 140(1):131-137. (Clone-specific: ELISA, Neutralization). View Reference
-
Abrams JS, Roncarolo MG, Yssel H, Andersson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992; 127:5-24. (Clone-specific: ELISA, Neutralization). View Reference
-
Cockayne DA, Muchamuel T, Grimaldi JC, et al. Mice deficient for the ecto-nicotinamide adenine dinucleotide glycohydrolase CD38 exhibit altered humoral immune responses. Blood. 1998; 92(4):1324-1333. (Clone-specific: ELISA, Neutralization). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology: IC/FCM Block). View Reference
-
Sander B, Hoiden I, Andersson U, Moller E, Abrams JS. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. Cytokine detection by immunoassay and intracellular immunostaining. J Immunol Methods. 1993; 166(2):201-214. (Clone-specific: ELISA). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.