Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


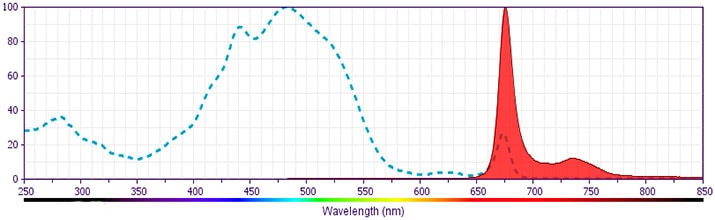
CD19 PerCP
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
The antibody reagent is stable until the expiration date shown on the label when stored at 2° to 8°C. Do not use after the expiration date. Do not freeze the reagent or expose it to direct light during storage or incubation with cells. Keep the outside of the reagent vial dry.
Do not use the reagent if you observe any change in appearance. Precipitation or discoloration indicates instability or deterioration.
CD19 is intended for in vitro diagnostic use in the identification of cells expressing CD19 antigen, using a FACS™ brand flow cytometer. The flow cytometer must be equipped to detect light scatter and the appropriate fluorescence, and be equipped with appropriate analysis software for data acquisition and analysis. Refer to your instrument user’s guide for instructions.
Development References (17)
-
Cabezudo E, Carrara P, Morilla R, Matutes E. Quantitative analysis of CD79b, CD5 and CD19 in mature B-cell lymphoproliferative disorders. Haematologica. 1999; 84(5):413-418. (Biology).
-
Centers for Disease Control. Guidelines for the performance of CD4+ T-cell determination in persons with human immunodeficiency virus infection. MMWR. 1992; 41:1-17. (Biology).
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Lymphocytes: Approved Guideline. NCCLS document H42-A. 1998. (Biology).
-
Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Peripheral Blood Lymphocytes. NCCLS document H42-T. 1992. (Biology).
-
Dörken B, Möller P, Pezzutto A, Schwartz-Albiez R, Moldenhauer G. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. New York, NY: Oxford University Press; 1989:34-36.
-
Evans RL, Wall DW, Platsoucas CD, et al. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to TH2 antigen. Proc Natl Acad Sci U S A. 1981; 78(1):544-548. (Biology). View Reference
-
Jackson AL, Warner NL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clincial Laboratory Immunology, Third Edition. Washington DC: American Society for Microbiology; 1986:226-235.
-
Kotzin BL, Benike CJ, Engleman EG. Induction of immunoglobulin-secreting cells in the allogeneic mixed leukocyte reaction: regulation by helper and suppressor lymphocyte subsets in man. J Immunol. 1981; 127(9):931-935. (Biology). View Reference
-
Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood. 1987; 70(5):1316-1324. (Biology). View Reference
-
Nadler LM. B Cell/Leukemia Panel Workshop: summary and comments. In: Reinherz EL, Haynes BF, Nadler LM, Bernstein ID, ed. Leukocyte Typing II: Human B Lymphocytes. New York: Springer-Verlag; 1986:3-43.
-
Nicholson J, Browning S, Orloff S, McDougal J. Inactivation of HIV-infected H9 cells in whole blood preparations by lysing/fixing reagents used in flow cytometry. J Immunol Methods. 1993; 160:215-218. (Biology).
-
Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture–Fourth Edition; Approved Standard. NCCLS document H3-A4. 1998. (Biology).
-
Protection of Laboratory Workers from Infectious Disease Transmitted by Blood, Body Fluids, and Tissue: Tentative Guideline. NCCLS document M29-T2. (Biology).
-
Reichert T, DeBruyere M, Deneys V, et al. Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol. 1991; 60(2):190-208. (Biology). View Reference
-
Tedder TF, Zhou LJ, Engel P. The CD19/CD21 signal transduction complex of B lymphocytes. Immunol Today. 1994; 15(9):437-442. (Biology). View Reference
-
Tursz T, Brouet J-C, Flandrin G, Danon F, Clauvel J-P, Seligmann M. Clinical and pathologic features of Waldenström's macroglobulinemia in seven patients with serum monoclonal IgG or IgA. Am J Med. 1977; 63:499-502. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For In Vitro Diagnostic Use.
23-22942-00
![]() Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.
Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.