-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- New Zealand (English)
-
Change country/language
Old Browser
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Human Regulatory T Cell Sorting Kit
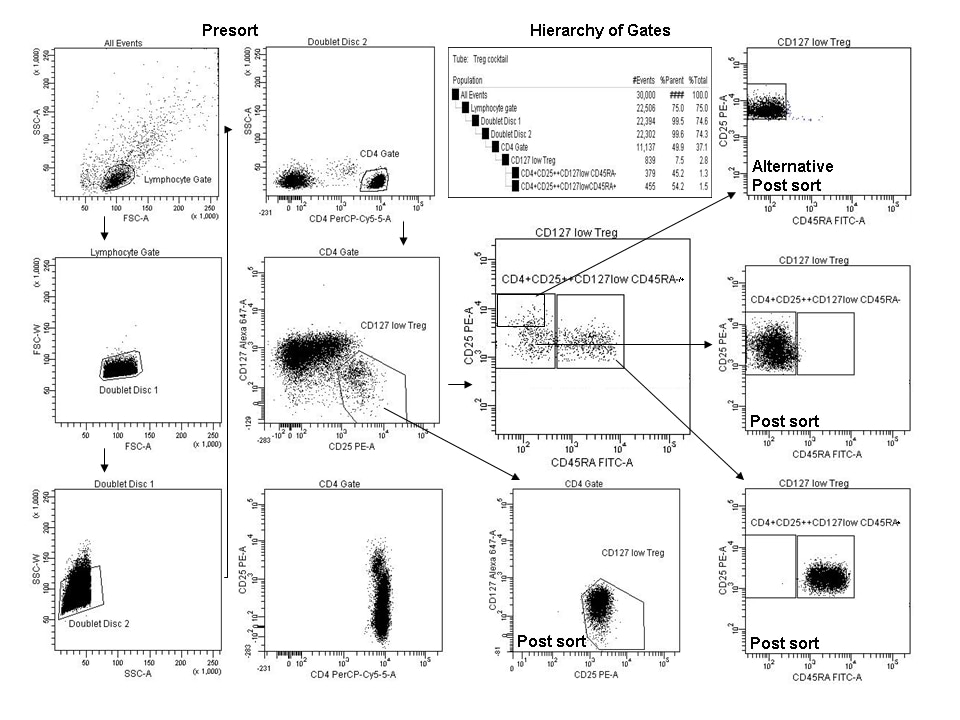
Example Sort Data. Example data was derived from PBMC isolated from sodium heparin anticoagulated whole blood. Cell viability was > 94% and cells were stained at 100 million cells/mL with the BD Human Regulatory T cell Sorting Kit. The stained cells were sorted on a FACSAria™ flow cytometer using FACSDiva™ software.
Presort: Lymphocytes were gated for expected morphology using a FSC-A vs. SSC-A \"Lymphocyte Gate\" followed by doublet discrimination using FSC-W and SSC-W parameters. Next, CD4+ lymphocytes were gated followed by gating of the CD25highCD127low T regulatory (Treg) cells.This population of CD4+CD25highCD127low can be directly sorted as shown. Alternately, CD45RA- and CD45RA+ staining patterns can provide an additional interrogation parameter used to identify CD4+CD25highCD127lowCD45RA+ and CD4+CD25highCD127lowCD45RA- populations (as shown). Cells were sorted in the purity mode using a 70 micron nozzle with a rate of 8,000-to-11,000 events per second.
Post Sort: Each sorted fraction was counted, divided into two fractions, and washed and suspended in culture media or wash buffer for cell culture or analysis respectively. At least 30,000 cells were suspended in 300 µL of wash buffer for purity measurements. Slight adjustments of gates were required to properly visualize the sorted fractions.
The flexibility of the kit allows for many sorting configurations. CD25 expression can vary depending upon donor and the application. An alternative gating strategy isolating CD45RA-CD25high activated T regs is shown in the upper right hand corner.


Example Sort Data. Example data was derived from PBMC isolated from sodium heparin anticoagulated whole blood. Cell viability was > 94% and cells were stained at 100 million cells/mL with the BD Human Regulatory T cell Sorting Kit. The stained cells were sorted on a FACSAria™ flow cytometer using FACSDiva™ software.
Presort: Lymphocytes were gated for expected morphology using a FSC-A vs. SSC-A \"Lymphocyte Gate\" followed by doublet discrimination using FSC-W and SSC-W parameters. Next, CD4+ lymphocytes were gated followed by gating of the CD25highCD127low T regulatory (Treg) cells.This population of CD4+CD25highCD127low can be directly sorted as shown. Alternately, CD45RA- and CD45RA+ staining patterns can provide an additional interrogation parameter used to identify CD4+CD25highCD127lowCD45RA+ and CD4+CD25highCD127lowCD45RA- populations (as shown). Cells were sorted in the purity mode using a 70 micron nozzle with a rate of 8,000-to-11,000 events per second.
Post Sort: Each sorted fraction was counted, divided into two fractions, and washed and suspended in culture media or wash buffer for cell culture or analysis respectively. At least 30,000 cells were suspended in 300 µL of wash buffer for purity measurements. Slight adjustments of gates were required to properly visualize the sorted fractions.
The flexibility of the kit allows for many sorting configurations. CD25 expression can vary depending upon donor and the application. An alternative gating strategy isolating CD45RA-CD25high activated T regs is shown in the upper right hand corner.

Example Sort Data. Example data was derived from PBMC isolated from sodium heparin anticoagulated whole blood. Cell viability was > 94% and cells were stained at 100 million cells/mL with the BD Human Regulatory T cell Sorting Kit. The stained cells were sorted on a FACSAria™ flow cytometer using FACSDiva™ software.
Presort: Lymphocytes were gated for expected morphology using a FSC-A vs. SSC-A \"Lymphocyte Gate\" followed by doublet discrimination using FSC-W and SSC-W parameters. Next, CD4+ lymphocytes were gated followed by gating of the CD25highCD127low T regulatory (Treg) cells.This population of CD4+CD25highCD127low can be directly sorted as shown. Alternately, CD45RA- and CD45RA+ staining patterns can provide an additional interrogation parameter used to identify CD4+CD25highCD127lowCD45RA+ and CD4+CD25highCD127lowCD45RA- populations (as shown). Cells were sorted in the purity mode using a 70 micron nozzle with a rate of 8,000-to-11,000 events per second.
Post Sort: Each sorted fraction was counted, divided into two fractions, and washed and suspended in culture media or wash buffer for cell culture or analysis respectively. At least 30,000 cells were suspended in 300 µL of wash buffer for purity measurements. Slight adjustments of gates were required to properly visualize the sorted fractions.
The flexibility of the kit allows for many sorting configurations. CD25 expression can vary depending upon donor and the application. An alternative gating strategy isolating CD45RA-CD25high activated T regs is shown in the upper right hand corner.


BD Pharmingen™ Human Regulatory T Cell Sorting Kit

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
The immune system protects individuals from a broad range of pathogenic microorganisms while avoiding inappropriate or extreme immune responses, such as autoimmune or allergic immune responses that could be harmful. Adaptive immunity is largely mediated by T and B lymphocyte populations that can specifically recognize a diverse constellation of foreign antigens. Through processes of rapid clonal expansion and differentiation, antigen-stimulated lymphocytes generate a vast array of potent effector and memory cell progeny to successfully rid the body of the offending foreign antigen.
A distinct class of T cells, called regulatory T cells (Treg), safeguards against the immune system's responsiveness to self antigens and restrains excessive responsiveness to foreign antigens that could be harmful to the host. Natural CD4+ T regulatory cells (nTreg) are generated in the thymus as a functionally mature subpopulation of T cells. These cells emigrate to the peripheral T cell pool and constitutively express medium to high levels of CD25 (IL-2 receptor α chain) and Foxp3 (forkhead box P3). The transcription factor Foxp3 is a member of the forkhead/winged-helix family of transcription factors and is a master regulator of Treg development and function. CD127 (IL-7 receptor α chain) constitutes another useful cell surface marker for identifying Treg cells. CD127 is downregulated on Treg cells, inversely correlating with the expression of Foxp3. Inducible CD4+ T regulatory cells (iTreg) can arise from mature peripheral naïve CD4+ T cells not expressing CD25 and Foxp3 (CD4+CD25-Foxp3- cells).
Recent studies indicate that natural human CD4+FoxP3+ T cells can be subdivided into phenotypically- and functionally-distinct subsets based on the coexpressed levels of Foxp3, CD25 and CD45RA. The CD45RA marker is particularly useful for identifying resting CD4+ natural Treg cells (CD45RA+) as well as in vivo-activated CD4+ Treg (CD45RA-) when used with Foxp3, CD25, and CD127 markers. Phenotypic and functional studies using these markers provide a basis for high resolution analyses of the dynamics of Treg cell generation and differentiation in development, age and disease states.
The BD™ Human Regulatory T Cell Sorting Kit provides reagents that allow investigators to stain viable human peripheral blood mononuclear cells (PBMC) or an enriched CD4+ T cell fraction of PBMC and to purify CD4+CD25+CD127lo cells (optional CD45RA+). These cells can then be tested functionally or used as precursors for expansion and differentiation cultures to generate activated Treg cells. Other cellular fractions such as CD4+CD25-CD127+ cells (naïve CD4+ T cells) can simultaneously be purified by flow cytometric sorting for subsequent functional characterization. In addition, fluorescent anti-human CD45RA antibody is included to facilitate further characterization or sorting of naïve (CD45RA+) or activated/memory (CD45RA-) CD4+Foxp3+ or CD4+Foxp3- T lymphocytes.
Preparation And Storage
Recommended Assay Procedures
Isolate Peripheral Blood Mononuclear Cells (PBMC)
Prepare PBMC by density gradient centrifugation.
Antibody staining for cell sorting
The following procedure describes how to stain the controls and cell sample for sorting. The kit does not require the use of isotype controls to perform a successful sort. Each population has been well characterized and verified for staining patterns. However, you may wish to use your own fluorescence minus one (FMO) isotype controls as desired.
Note: Follow aseptic stain and sort procedures if planning to expand isolated Treg population.
1. Label five 12 × 75-mm tubes for compensation. Follow instructions from Cat. No. 552843 Anti-Mouse Ig, κ/Negative Control (FBS) Compensation Particles Set. Prepare beads for flow cytometry per the recommended assay procedure.
Tube 1: Unstained Compensation beads
Tube 2: FITC Compensation beads
Tube 3: PE Compensation beads
Tube 4: PerCP-Cy5.5 Compensation beads
Tube 5: AlexaFluor® 647 Compensation beads
Tube 6: Optional: Horizon V450 compensation tube for post sort FoxP3 staining
2. Add the following antibodies from the single vials contained in the kit to the tubes labeled in Step #1.
-FITC Compensation tube: 20 µl of CD45RA FITC
-PE Compensation tube: 20 µl of CD25 PE
-PerCP-Cy5.5 Compensation tube: 20 µl of CD4 PerCP-Cy5.5
-AlexaFluor® 647 Compensation tube: 20 µl of CD127 APC
Prepare beads for flow cytometry as described in the instructions for Cat. No. 552843, Anti-Mouse Ig, κ/Negative Control (FBS) Compensation Particles Set.
3. Stain Sample: Aliquot the sample to be sorted into a 50 ml tube for staining the PBMC or CD4 enriched cells. For total PBMC numbers less than 100 million the staining concentration is 20 million per 1.0 ml. For total PBMC numbers greater than 100 million, the staining concentration is 100 million per 1.0 ml.
Note: Test size equals 200 µl per 100 million cells. The wash buffer is 1x PBS + 1% Human AB serum.
4. Add antibody: 200 µl each of Cocktail and CD45RA per 100 million cells. For example:
-120 million cells, add 240 µl of Cocktail and CD45RA to a final volume of 1.2 ml
- 50 million cells, add 100 µl of Cocktail and CD45RA to a final volume of 2.5 ml
5. Incubate protected from light at 18°C - 22°C for 30 min.
6. Add enough wash buffer (e.g. 49 ml) to fill 50 ml tube and centrifuge at 250 x g for 15 min.
7. Aspirate the supernatant and resuspend the sample at a concentration of 5 - 10 million cells/ml in wash buffer.
8. Prepare reception tubes. Coat 12 × 75-mm polypropylene tubes with 0.2 ml of human AB serum, invert, then add 0.2 ml of media [(formulation; X-VIVO-15 (Cambrex) + 10% Human AB serum (Sigma) + 0.2% Acetylcysteine solution)] or use PBS wash buffer.
9. Use the FACSAria™ cell sorter to isolate the cell populations of interest.
Population options for sorting:
Core population CD4+/CD25int-hi/CD127lo
CD4+/CD25int-hi/CD127lo/CD45RA+ cells
CD4+/CD25int-hi/CD127lo/CD45RA- cells
CD4+/CD25-/CD127lo
CD4+/CD25-/CD127lo/CD45RA+ cells,
CD4+/CD25-/CD127lo/CD45RA- cells.
10. Collect fractions in 12 × 75-mm polypropylene tubes coated with human AB serum, and containing media.
11. Centrifuge fractions at 250 x g for 10 minutes, remove supernatant without disturbing the pellet. Resuspend in wash buffer and recover at least 30,000 cells for FoxP3 staining or post sort acquisitions, and/or at least 50,000 cells for expansion.
Note: be sure to use 1x PBS + 1% Human AB serum when acquiring post sort fractions.
12. Proceed with modified FoxP3 staining protocol (below), expansion, suppression, or other downstream protocol of choice.
Sorting Protocol. A 70 or 100 micron nozzle may be used for this procedure. A 70 micron nozzle will allow for flow rates and pressures high enough to sort ~100 million PBMC in 0.5-2.5 hours to obtain ~90,000 CD45RA+ T regulatory cells depending upon the donor. A 100 micron nozzle may allow for higher purities and viabilities while approximately doubling the sort time. Sorting of 200 million cells or more may be required for adequate recoveries of CD25brightCD45RA- T cells. See figure and figure legend for complete gating guidelines.
FoxP3 post sort staining protocol. The following procedure describes a modified FoxP3 staining protocol for post sort fractions. Handle cells minimally and avoid high g forces after sorting. A successful sort of 100-250 million PBMC will provide enough cells to test for viability and FoxP3 expression in a verification experiment and likely provide enough cells for expansion. However, we recommend conserving CD45RA fractions for purity measurements and stain cells for FoxP3 when cells are plentiful later in expansion. We recommend staining in small polypropylene tubes or plates. Surface staining is not required if post sort fractions are fixed and tested the day of the sort, but FITC fluorescence signal to noise will be reduced by this procedure. Alternatively, costain expanded cells with CD3 (Cat. No. 555333) and/or CD4 (Cat. No. 565999), Human Regulatory T Cell Cocktail (Cat. No. 560249) or stain with 10 µl of this kit per test.
1. Bring the buffers to RT before use. Prepare working solutions of the BD Pharmingen Human FoxP3 Buffer Set
(Cat. No. 560098) and follow this modified protocol:
2. Label five polypropylene 0.65 ml microcentrifuge tubes (VWR MN 87003-290)
Tube 1: Pre sort fraction
Tube 2: CD4+CD25++CD127lowCD45RA- Horizon V450 isotype control (Cat. No. 560373)
Tube 3: CD4+CD25++CD127lowCD45RA- Horizon V450 FoxP3 (Cat. No. 560460)
Tube 4: CD4+CD25++CD127lowCD45RA+ Horizon V450 isotype control
Tube 5: CD4+CD25++CD127lowCD45RA+ Horizon V450 FoxP3
3. Prepare human sorted fractions for FoxP3 staining.
4. Centrifuge the receiving tubes after the sort 250 x g for 10 minutes, gently remove wash buffer.
5. Dilute cells to at least 30,000 cells per 50 µl in BD Pharmingen Stain Buffer (FBS).*
Note: use 200,000 cells for Tube 1 to assist in adjusting FSC and SSC when acquiring fixed cells.
6. Add 500 µl of BD Pharmingen Stain Buffer (FBS). Centrifuge 250 x g for 5 minutes, gently remove wash buffer.
7. To fix cells, add 250 µl of 1x Buffer A per tube, vortex gently for one second, incubate for 10 minutes at RT protected from light.
Note: Optional step, store overnight at 4°C and continue procedure the next day. Bring to RT before step 8.
8. Centrifuge 250 x g for 5 minutes, and remove fixative. Caution: Be aware the pellet is buoyant.
9. To permeabilize the cells, gently re-suspend pellet in residual volume fix buffer and then add 250 µl of a 1x working solution of Human FoxP3 Buffer C to each tube. Vortex gently for one second, incubate for 30 minutes at RT protected from light.
10. Add 500 µl of BD Pharmingen Stain Buffer (FBS). Centrifuge 250 x g for 5 minutes, gently remove wash buffer leaving behind ~50 µl of residual buffer.
11. Add V450 conjugated FoxP3 antibody and/or isotype control at 0.5x test size indicated on the vial. Vortex gently for one second.
12. Incubate for 30 minutes in the dark at RT.
13. Repeat wash step #9.
14. Resuspend in 300 µl 1x PBS and analyze immediately. Acquire 500 to 2,000 CD4 positive cell events for best results.
Note: Optimize FSC vs. SSC settings with pre-sort fraction. Carryover from tube to tube can affect interpretation of data collected on very small fractions of cells. It is advised to acquire a tube of PBS between runs to define background and/or carryover from previous tubes in the run.
* We recommend using the BD Pharmingen Stain Buffer (FBS; Cat No. 554656) for initial surface staining and all wash steps and covering tubes during incubation steps with caps or parafilm. We also recommend optimizing forward scatter and side scatter voltages to visualize lymphocytes as separate from debris and/or platelets before acquisition.
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cell and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Alexa Fluor® 647 fluorochrome emission is collected at the same instrument settings as for allophycocyanin (APC).
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- An isotype control should be used at the same concentration as the antibody of interest.
- This product is provided under an intellectual property license between Life Technologies Corporation and BD Businesses. The purchase of this product conveys to the buyer the non-transferable right to use the purchased amount of the product and components of the product in research conducted by the buyer (whether the buyer is an academic or for-profit entity). The buyer cannot sell or otherwise transfer (a) this product (b) its components or (c) materials made using this product or its components to a third party or otherwise use this product or its components or materials made using this product or its components for Commercial Purposes. Commercial Purposes means any activity by a party for consideration and may include, but is not limited to: (1) use of the product or its components in manufacturing; (2) use of the product or its components to provide a service, information, or data; (3) use of the product or its components for therapeutic, diagnostic or prophylactic purposes; or (4) resale of the product or its components, whether or not such product or its components are resold for use in research. For information on purchasing a license to this product for any other use, contact Life Technologies Corporation, Cell Analysis Business Unit Business Development, 29851 Willow Creek Road, Eugene, OR 97402, USA, Tel: (541) 465-8300. Fax: (541) 335-0504.
- Cy is a trademark of GE Healthcare.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Alexa Fluor™ is a trademark of Life Technologies Corporation.
- Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
- Please observe the following precautions: We recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to protect exposure of conjugated reagents, including cells stained with those reagents, to any room illumination. Absorption of visible light can significantly affect the emission spectra and quantum yield of tandem fluorochrome conjugates.
Data Sheets
Companion Products



Recently Viewed
| Description | Quantity/Size | Part Number | EntrezGene ID |
|---|---|---|---|
| Human Regulatory T Cell Sorting Cocktail | 10 Tests (1 ea) | 51-9006625 | N/A |
| FITC Mouse anti-Human CD45RA | N/A | 51-9006623 | N/A |
| Alexa Fluor®647 Mouse anti-Human CD127 | N/A | 51-9006624 | N/A |
| PE Mouse anti-Human CD25 | N/A | 51-9006622 | N/A |
| PerCP-Cy™5.5 Mouse anti-Human CD4 | N/A | 51-9006621 | N/A |
Development References (7)
-
Baecher-Allan, C, Viglietta, V, Hafler, DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004; 16(2):89-98. (Biology). View Reference
-
Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006; 108(13):4260-4267. (Biology). View Reference
-
Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006; 203(7):1701-1711. (Biology). View Reference
-
Miyara, M, Yoshioka, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009; 30(6):899-911. (Biology). View Reference
-
Sakaguchi, S, Yamaguchi, et al. Regulatory T cells and immune tolerance. Cell. 2008; 133(5):775-787. (Biology). View Reference
-
Seddiki N, Santner-Nanan B, Martinson J et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006; 203(7):1693-1700. (Biology). View Reference
-
Ziegler, SF. FOXP3: of mice and men. Annu Rev Immunol. 2006; 209-226. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
