-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

B Cells
For Professionals in Research
B lymphocytes or B cells are a subset of adaptive immune cells that start their maturation in the fetal liver and postnatal bone marrow. B cells are known for their ability to support humoral immunity through the production of antibodies, but they carry other key functions such as phagocytosis and antigen presentation. Cells committed to the B cell lineage express CD19 and other markers evolve during their maturation and differentiation.1 B cells remain an active area of research because they play a critical role in the immune system. Perturbations in B cell development or function are implicated in several disease states. Understanding the basis of B cell function and dysfunction is of particular interest for the development of new vaccines and therapies. From intracellular and surface markers to secreted proteins and functional assays, BD Biosciences offers the latest reagents and methods to study B cell biology in health and disease.
How do B cells develop?
B cells pass through a number of developmental stages both before and after exposure to antigens. As they mature and differentiate, they give rise to multiple functionally distinct subsets. Differential expression of cell surface and intracellular markers, as well as their distinct immunoglobulin and cytokine secretion profiles, provide valuable clues to the diverse nature and function of the different B cell subsets. For example, the expression of syndecan-1 (CD138) distinguishes circulating plasmablasts and plasma cells, the “professional” antibody-secreting B cells, from other developmental and functional subsets. Failure of successful completion of developmental processes will induce apoptosis in maturing B cells. To support the use of multicolor flow cytometry for the study of B cells, BD offers a wide portfolio of reagents for B cell phenotyping. They are available in multiple formats, to provide maximum flexibility in panel design. We continue to add new specificities as new markers emerge. The schematic summarizes the main developmental and differentiation stages as well as some of the key markers associated with each B cell subset in mouse and human.
Conventional B cell maturation
B cells originate from hematopoietic stem cells (HSCs) located in bone marrow, where they pass through the first stages of development. The still immature B cells then migrate to secondary lymphoid tissues, where most of them continue their development into mature follicular B cells. When the B cell receptor (BCR) complex of a mature B cell, consisting of the membrane-bound (m) forms of IgM and IgD, binds its cognate foreign antigen, the cell becomes activated and differentiates into an antibody-secreting plasma cell.2
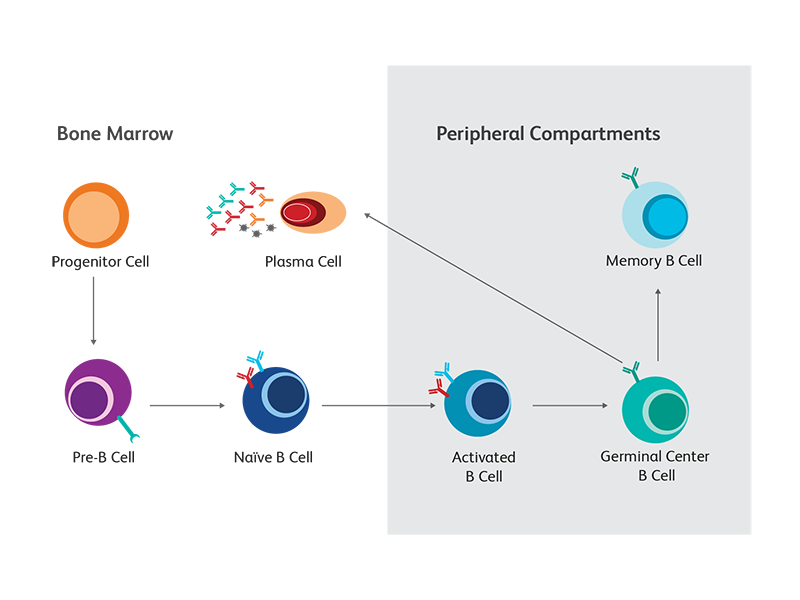
B cell subsets with different functions
B cells also follow alternative differentiation pathways from those of conventional B cells, resulting in subsets that have distinct functions and marker expression patterns. For example, marginal-zone (MZ) B cells function as innate-like cells. Unlike conventional B cells, they can be activated through Toll-like receptor (TLR)-ligation, bypassing the BCR. They also tend to express CD1d and CD21 but not CD23. B cell subsets with regulatory function have been identified and are distinguished by their ability to secrete IL-10 or TGF-β-1.3
Analysis of B cell maturation
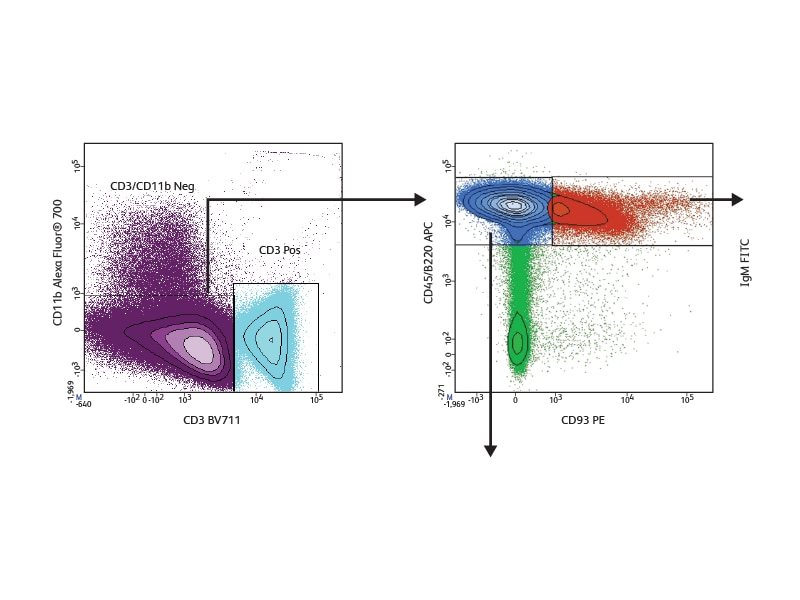
With a comprehensive selection of antibodies to mouse and human markers, BD can support a wide variety of phenotyping panels for the study of B cells across all developmental stages. To illustrate the use of differential marker expression for B cell analysis, seven cell surface markers were used to analyze B cell subsets in mouse bone marrow, allowing discrimination of seven different developmental phases in this tissue. Pre-pro-B, Pro-B and Pre-B cells could be distinguished within the low positive CD45R/B220 population based on their differential expression of BP1 and CD24. Immature, transitional and early and late mature B cells could be segregated based on differential expression of IgM and IgD. The expression of the IL-7 receptor, CD127, was analyzed in these different subsets and was shown to decrease as B cells matured.
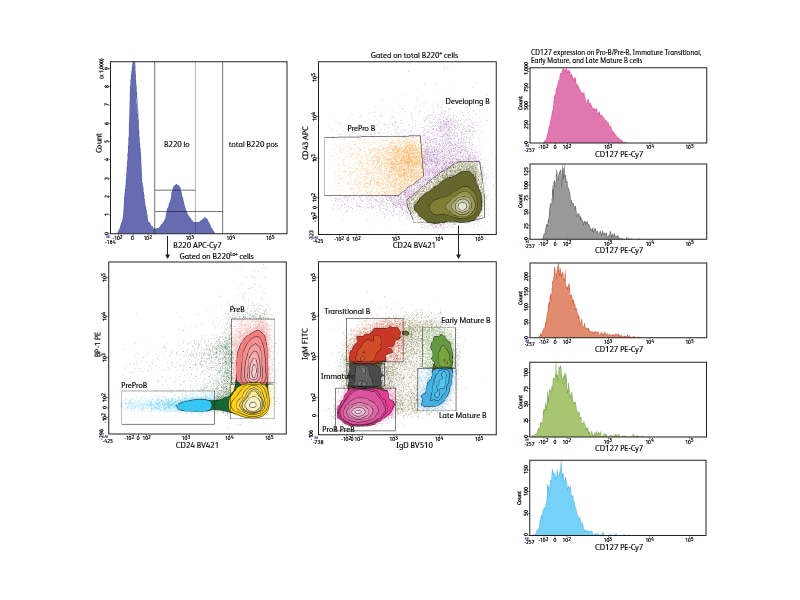
References
- Martínez-Riaño A, Bovolenta ER, Mendoza P, et al. Antigen phagocytosis by B cells is required for a potent humoral response. EMBO Rep. 2018;19(9):e46016. doi:10.15252/embr.201846016
- Yam-Puc JC, Zhang L, Zhang Y, Toellner KM. Role of B-cell receptors for B-cell development and antigen-induced differentiation. F1000Res. 2018;7:429. doi:10.12688/f1000research.13567.1
- Matsushita T. Regulatory and effector B cells: Friends or foes? J Dermatol Sci. 2019;93(1):2-7. doi:10.1016/j.jdermsci.2018.11.008
Types of B cells
Plasma cells
B cells are well known for their ability to produce antibodies that are vital to neutralize foreign entities or infected cells. Antibody secreting cells (ASCs), which include plasmablasts and plasma cells, lack the CD20 B cell marker and in humans express a combination of CD27, CD38 and CD138. Plasmablasts act early during adaptive immune responses, produce low-affinity immunoglobulins and have a short life. Plasma cells are more differentiated and comprise a short-lived and a long-lived subset. Short-lived plasma cells are similar to plasmablasts in their lifespan and antibody secretion rate and while plasmablasts express all immunoglobulin (Ig) isotypes, plasma cells have a preference for IgM and IgG. Long-lived plasma cells have the longest lifespan and the highest rate of antibody secretion with immunoglobulin (Ig) isotypes being IgG > IgA > IgM.4
Effector B cells
Effector B cells or B-eff play a supportive role to immune responses through antigen presentation and secrete an array of cytokines (e.g., IL-2, IL-6, GM-CSF, IL-12, IL-17, IFN-γ, TNF-α) to induce activation of macrophages (e.g., interferon-gamma), T cell differentiation (IL-6 for Th17) and plasma cell differentiation for antibody production.3 Effector B cells also contribute to unregulated immune responses by supporting plasma cell differentiation for autoantibody production in autoimmune diseases such as systemic lupus erythematosus (SLE) and multiple sclerosis (MS). In MS, CD138+ B cells have been shown to accumulate in the cerebrospinal fluid (CSF) and contribute in maintaining chronic inflammation in the CNS through inflammatory cytokine secretion and resident microglia activation.5
Regulatory B cells
As B cells secrete cytokines to regulate the functions of immune cells, they also receive environmental signals from immune counterparts that regulate their functions. In autoimmunity, a balance between effector B cells and regulatory B cells (B-reg) can be induced by macrophage and dendritic cell-derived BAFF. In SLE, excess BAFF promotes autoantibody production by plasma cells while inhibiting activation of IL-10 producing B-regs. Most of these IL-10 secreting regulatory B cells can be identified by CD9 (88%) and tumor necrosis factor receptor 2 (TNFR2) expression.3,6
References
- Tellier J, Nutt SL. Plasma cells: The programming of an antibody-secreting machine. Eur J Immunol. 2019;49(1):30-37. doi:10.1002/eji.201847517
- Matsushita T. Regulatory and effector B cells: Friends or foes? J Dermatol Sci. 2019;93(1):2-7. doi:10.1016/j.jdermsci.2018.11.008
- Knier B, Hiltensperger M, Sie C, et al. Myeloid-derived suppressor cells control B cell accumulation in the central nervous system during autoimmunity. Nat Immunol. 2018;19(12):1341-1351. doi:10.1038/s41590-018-0237-5
- Fillatreau S. Regulatory functions of B cells and regulatory plasma cells. Biomed J. 2019;42(4):233-242. doi:10.1016/j.bj.2019.05.008
Assays to assess B cell functions
Antibody production
Measurement of the types and amounts of immunoglobulins and cytokines that are secreted by B cells provides insight into the quality and quantity of their immune response—features that are also frequently altered in disease states. Methods that allow multiplexed measurements are increasingly being used to measure immunoglobulins (Igs) and cytokines. In addition to the benefits of multiplex analysis, they have several unique advantages compared to classically used techniques.
BD® Cytometric Bead Array: multiplexed quantitation
BD® Cytometric Bead Array (CBA) is a bead-based immunoassay that can simultaneously quantify multiple analytes from the same sample. The BD® CBA Human Immunoglobulin Flex Set system provides ready-to-use reagents that serve as building blocks for the multiplexed quantitation of multiple Ig subclasses. Because the amount and type of antibody in a particular immune response can vary greatly, measurements of these parameters can provide insight into the response after immunization or vaccination or serve as an indicator to assess immunoglobulin deficiency disorders. For example, BD® CBA assays have been used to analyze the Igs and cytokines secreted by B cells during inflammation.7
Cytokine production
B cells secrete a range of different cytokines based on their state of activation.
| Cell Type | Cytokines Secreted |
|---|---|
| B effector 1 (Be-1) | IFN-γ, IL-12, TNF |
| B effector 2 (Be-2) | IL-2, IL-4, IL-6. TNF |
| Regulatory B | IL-10, TGF-β1 |
BD® CBA Assay for cytokines
BD® CBA Flex Sets for cytokines provide an open and configurable menu of bead-based reagents designed for easy and efficient multiplexing. The specificities include a wide range of human and mouse cytokines. Data comparing results using BD® CBA standards to the NIBSC/WHO International Standards are available as a guideline to facilitate comparisons of cytokine concentration values determined by different laboratories or methods. Measuring which cytokines are secreted can also provide information about the activity of different functional B cell subsets in a sample, such as B effector-1 or -2, or regulatory B cells (B-regs) secreting anti-inflammatory cytokines such as IL-10. BD® CBA Flex Sets have been used to quantitate the secretion of several B cell–related cytokines and chemokines after manipulation of exhausted tissue-like B-memory cells in chronic virally infected individuals, e.g., HIV and Plasmodium falciparum.8,9
Intracellular signaling
The development, activation and differentiation of B cells are accompanied by a number of changes in intracellular molecules, including transcription factors, phosphosignaling proteins and cytokines. BD offers solutions that uniquely enable analysis of these intracellular molecules to support researchers in deciphering the interconnected pathways regulating B cell biology. Multicolor flow cytometry is a powerful technique for the analysis of intracellular molecules. Simultaneous analysis with markers for specific cell subsets can reveal information about the differentiation and activation state of distinct populations of B cells. While techniques for cell surface staining are relatively standard, optimal staining for intracellular markers often depends on the biology of the target protein.
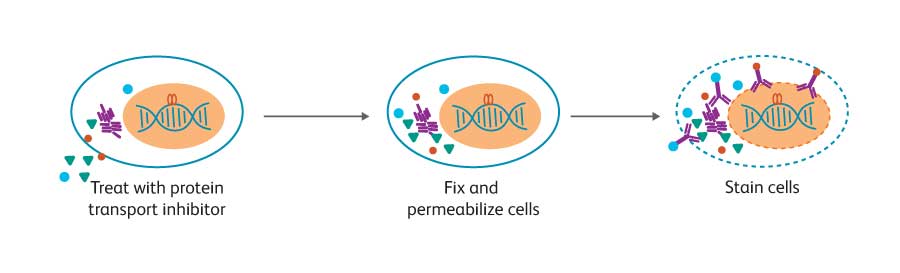
To facilitate the detection of these specific intracellular molecules by flow cytometry, BD has developed monoclonal antibodies, specialized buffer systems and kits. Flow cytometry has several advantages over commonly used methods such as western blotting —multiple parameters can be measured simultaneously from heterogeneous samples such as blood. This conserves precious samples and provides detailed results at the cellular level.
Bcl6 expression in mouse lymph node cells
Mouse lymph node cells were stained with anti CD45R/B220, CD4, IgD and CD95, fixed and permeabilized using the BD Pharmingen™ Transcription Factor Buffer Set, and stained with anti-Bcl-6 Alexa Fluor™ 488. Flow cytometry was performed using a BD® LSR II System. Bcl-6 expression was observed in germinal center B cells.
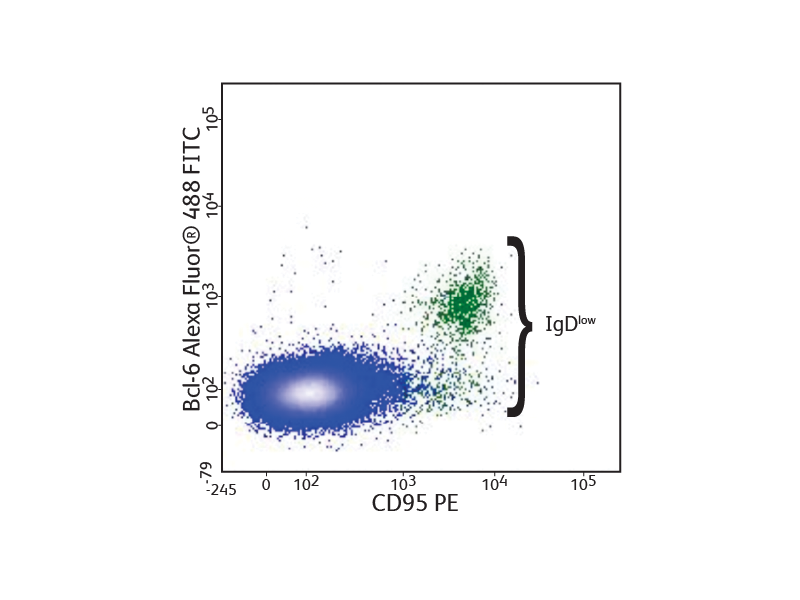
Detection of transcription factors
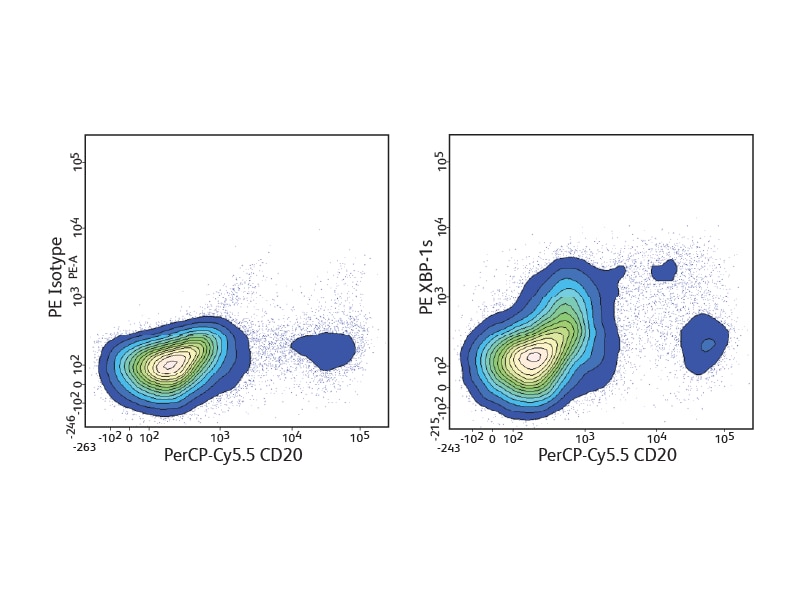
The BD Pharmingen™ Transcription Factor Buffer permeabilizes cells sufficiently to allow the exposure of intranuclear epitopes, while still being gentle enough to allow the detection of most cell surface proteins. It can be used alone or in combination with cell surface markers and cytokines for an improved detection of a number of different transcription factors. BD Biosciences offers several B cell–relevant, flow-validated transcription factor antibodies, including unique specificities. Some, such as Oct-2 and Pax-5, are expressed through the follicular or germinal center (GC) stages. Bcl-6 characterizes proliferating GC B cells, while others, such as Blimp-1 and XPB-1s, are typical for plasma cells.
Detection of phosphoproteins
BD Phosflow™ Antibodies are monoclonal phosphoepitope–specific antibodies validated for flow cytometric detection. The recommended permeabilization buffer for most BD Phosflow™ Antibodies is BD Phosflow™ Perm Buffer III, but alternative permeabilization buffers also are available to meet particular experimental needs.
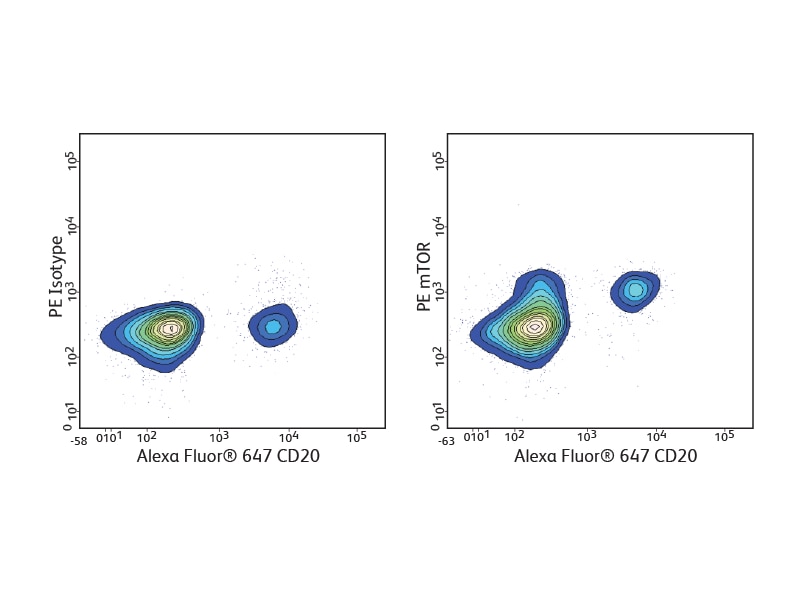
A number of BD Phosflow™ Antibody specificities are available for analysis of signaling pathways involved in B cell development and activation, including phospho-Akt, phospho-Btk, phospho-Syk, phospho-BLNK, phospho-CD20/BL-CAM, phospho-IKKγ, phospho-NFκB and phospho-mTOR.
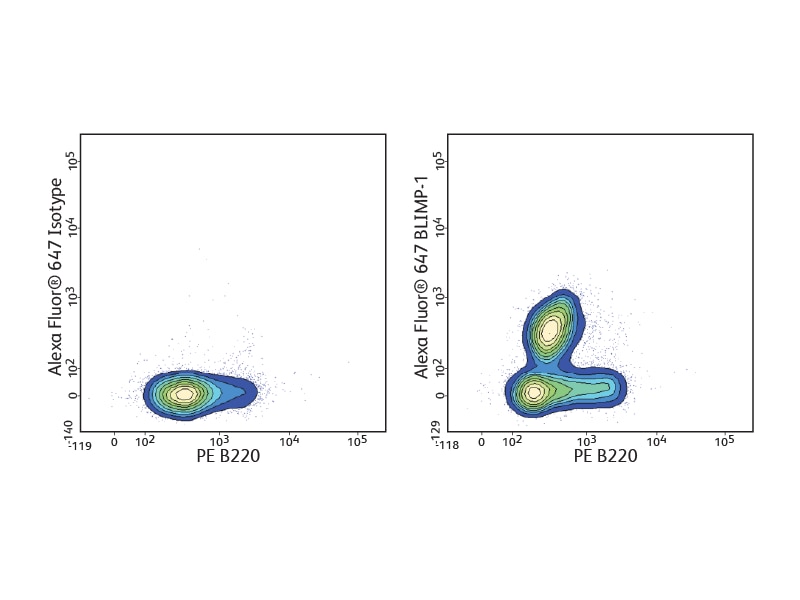
Detection of cytokines
Using a protein transport inhibitor to block secretion, researchers can detect cytokines in the cell in which they are being produced. This makes it possible to easily determine if the cytokine production by an activated cell population is the result of a few cells producing large amounts of cytokine or a large population of cells producing small quantities of cytokine per cell. BD kits, buffer systems and protocols for intracellular cytokine staining include BD Cytofix/ Cytoperm™ Fixation/Permeabilization Solution, which is suitable for staining most cytokines and cell surface markers. A wide selection of direct conjugates is available, covering cytokines often used to distinguish B-regs, and those secreted by B effector-1 (Be-1) and B effector-2 (Be-2) cells.
References
- Lu Y, Liu F, Li C, Chen Y, Weng D, Chen J. IL-10-producing B cells suppress effector T cells activation and promote regulatory T cells in crystalline silica-induced inflammatory response in vitro. Mediators Inflamm. 2017;2017:8415094. doi:10.1155/2017/8415094
- Kardava L, Moir S, Wang W, et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121(7):2614-2624. doi:10.1172/JCI45685
- Ambegaonkar AA, Kwak K, Sohn H, Manzella-Lapeira J, Brzostowski J, Pierce SK. Expression of inhibitory receptors by B cells in chronic human infectious diseases restricts responses to membrane-associated antigens. Sci Adv. 2020;6(30):eaba6493. doi:10.1126/sciadv.aba6493
Tools for B cell immunophenotyping
A multicolor immunophenotyping approach is well suited to the study of B cells since they, by nature, require the use of more markers than, for example, T cells, to define the basic subsets present in most samples.
Backbone markers
A wide variety of B cell studies employ cell surface markers that define the major B cell subsets, and these form the ‘backbone’, or core, for panels to do more detailed analysis. The key marker for B cell panels is the lineage marker CD19, which is expressed by almost all cells belonging to the B cell lineage. In the mouse, CD45R/B220 is traditionally used. Most panels also include surface-expressed IgD and IgM since the BCR isotype provides information about the differentiation stage of the B cells. To provide flexibility in panel design, BD offers all of these markers in a wide selection of formats.
Multicolor flow
When a panel of five markers is measured on two lasers, the fluorochromes that are typically used exhibit spill-over into other fluorescence channels, thus requiring compensation. Researchers who have access to an instrument with four or five lasers can use this capability to their advantage to simplify setup and minimize the need for compensation.
Instrument setup for multicolor flow
Instrument setup for multicolor experiments using antibody panels of more than four colors can be time consuming. Five-laser instruments are often equipped with a 355-nm UV laser, which is typically used for side population analysis, calcium measurement using indo-1 or viability staining.
To allow users to take full advantage of this equipment for phenotyping experiments, BD has developed a new UV-excited polymer-based dye, BD Horizon Brilliant™ Ultraviolet 395 (BUV395).
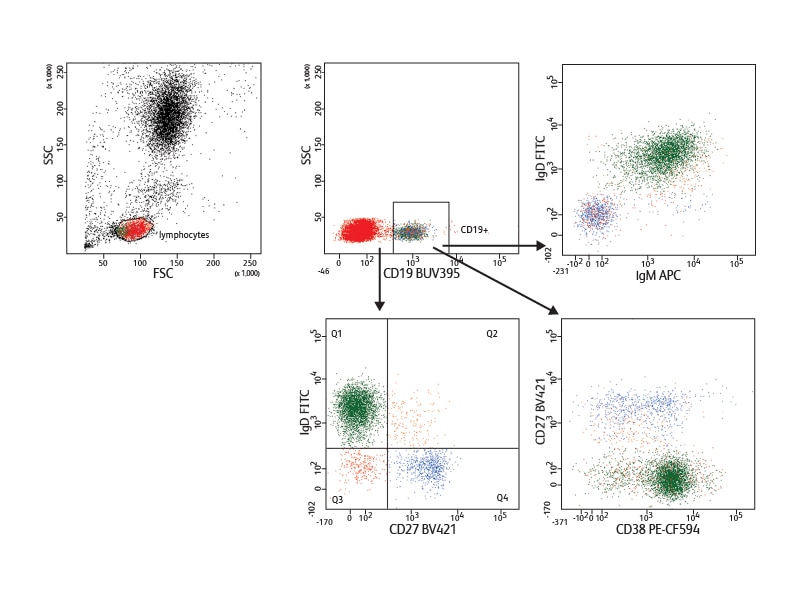
Incorporating antibodies conjugated to BUV395 into a panel in which each fluorochrome is excited by a different laser results in minimal or no spillover between them. This is illustrated by an example of a five-color panel discriminating naive and memory B cells. Using this panel, only minimal setup and virtually no compensation were needed.
In the example shown, nine cell surface markers were used to analyze B cell subsets in mouse spleen. They allowed discrimination between the different developmental phases present in this tissue. Follicular B I and II and marginal cell subsets (marginal zone and marginal zone progenitor) were identified based on expression of IgM, IgD, CD21 and CD23. The different levels of IgM and CD23 expression helped distinguish the stages of transitional subsets (T1, T2 and T3). Measurement of the expression of Pax-5 in the different subsets revealed differential Pax-5 expression in follicular and marginal zone B cells.
Combining measurements to maximize results
Advances in buffer systems and methodologies now make it easier to simultaneously measure both surface and intracellular markers and help researchers move toward information-rich, combined measurements. For example, several cell types in a heterogeneous sample of differentiating B cells can be monitored using a combination of cell surface and intracellular markers to efficiently get more relevant data out of each sample. The availability of antibodies to both B cell surface markers and specific transcription factors in many different conjugates, including new BD Horizon™ Dyes, adds flexibility when designing multicolor experiments.
Basic panel
Depending on the type of sample that is being analyzed, different markers can be selected to define the specific B cell subsets of interest. The example shown analyzes human peripheral blood using CD19, CD20, IgD, CD27, CD38 and CD24. With this six-color panel it is possible to identify transitional B cells (CD24high, CD38high), discriminate between naïve and memory cells (naïve cells are CD27–IgD+, memory cells CD27+IgD–), and identify plasmablasts (CD38+).1
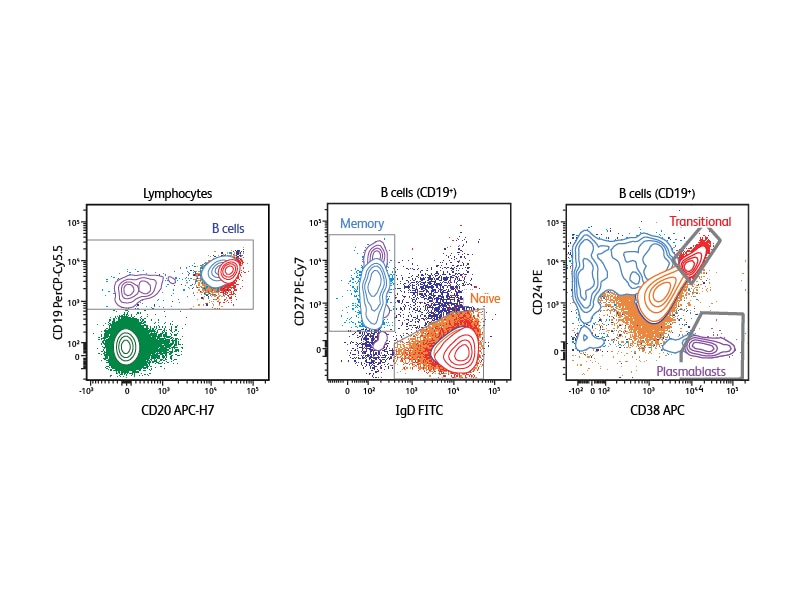
Deeper phenotyping
Because the phenotype of some subsets is very similar to others, often a more extensive phenotyping panel is required. For example, a panel might distinguish the many B cell developmental stages present in bone marrow B cells. Adding markers can also provide a more fine-detail analysis, for example, defining memory cells or different types of plasma cells. Other molecules offer insight into the potential for cells to home and localize within the body (chemokine receptors, CD62L). Adding activation markers (CD69, CD25, CD80, CD86) can inform which subsets are activated and provide important clues about an individual’s immune response.
Tools for building panels
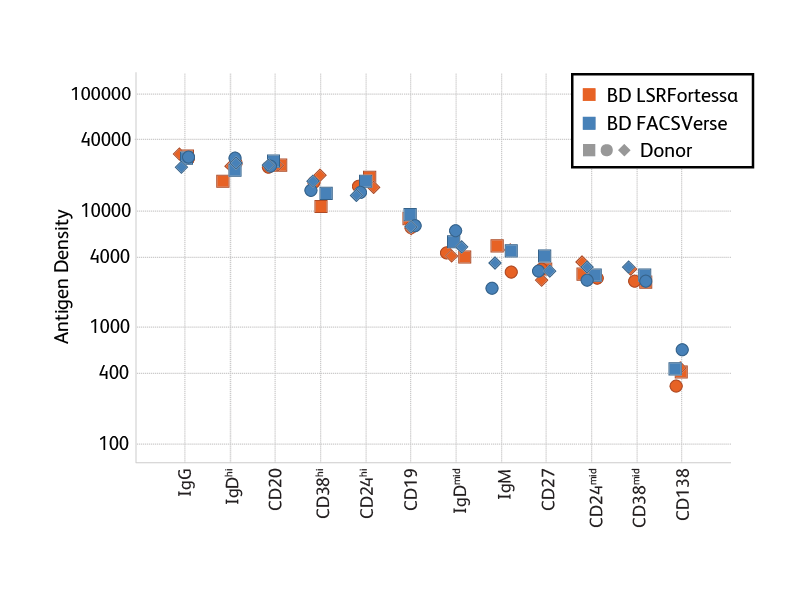
Antigen Density. When adding markers to a panel, researchers can take advantage of antigen-density information to optimize antigen-fluor selection. In general, we recommend choosing bright fluorochromes for markers with low antigen density and the less bright fluorochromes for highly expressed markers. To support researchers, BD is generating information about the antigen density of a number of markers on peripheral blood cells. Bright Conjugates for Dim Markers. Certain B cell populations are so rare, or defined by dimly expressed antigens, that achieving sufficient resolution is critical to obtaining good data in staining experiments. In particular for such rare or dim populations, the use of very bright fluorochromes helps improve resolution. A number of B cell marker antibodies are available conjugated to very bright BD Horizon Brilliant Violet™ (BV) Dyes, such as BV421, BV510 and BV605.
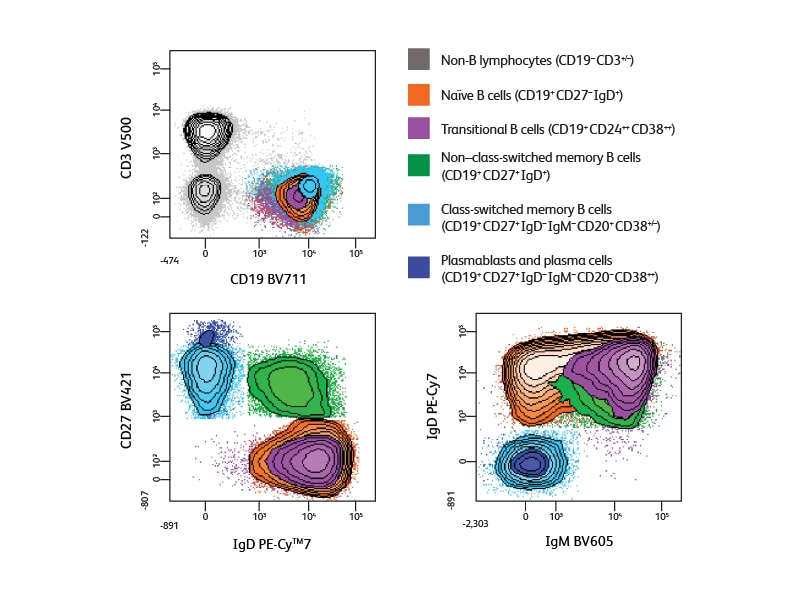
An extended panel for defining additional subsets
In designing the panel used for the 10-color analysis experiment shown, information about antigen density helped optimize the fluorochrome distribution. Bright fluorochromes were chosen for the low-density antigen CD138 and to allow resolution of populations expressing different levels of IgD and CD38, and the extremely bright BV dyes were used to obtain optimal detection of CD27 and IgM. Analysis using this panel provided the additional information about Ig subclass expression, allowing identification of both IgG+ and IgG– post-class-switched memory cells (IgM–IgD–CD38–CD27+/dim) and the clear discrimination of plasma cells (IgM–IgD–CD20–CD38++).
References
- Finak G, Langweiler M, Jaimes M, et al. Standardizing flow cytometry immunophenotyping analysis from the human immunophenotyping consortium. Sci Rep. 2016;6:20686. doi:10.1038/srep20686
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Alexa Fluor is a trademark of Life Technologies Corporation. CF is a trademark of Biotium, Inc. Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.