Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


CD8 (Leu™-2a) PE
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
1. For in vitro diagnostic use.
2. When stored at 2° to 8°C, the antibody reagent is stable until the expiration date shown on the label. Do not use after the expiration date.
3. The antibody reagent should not be frozen or exposed to direct light during storage or during incubation with cells. Keep the reagent vial dry.
4. Alteration in the appearance of the reagent, such as precipitation or discoloration, indicates instability or deterioration. In such cases, the reagent should not be used.
5. The antibody reagents contain sodium azide as a preservative; however, care should be taken to avoid microbial contamination, which may cause erroneous results.
Becton Dickinson Immunocytometry Systems (BDIS) CD8 (Leu™-2a) phycoerythrin (PE) is a single-color direct immunofluorescence reagent for enumerating percentages of mature human suppressor/cytotoxic (CD8+) lymphocytes in erythrocyte-lysed whole blood (LWB) or peripheral blood mononuclear cell (PBMC) suspensions.
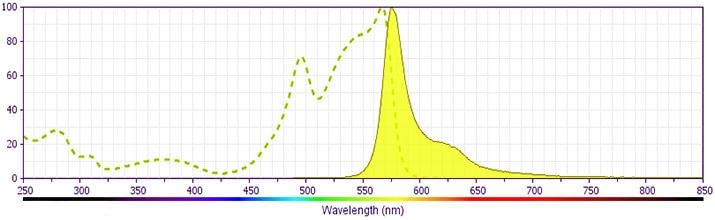
Development References (21)
-
A National Committee for Clinical Laboratory Standards. Procedures for the Collection of Diagnostic Blood Specimens by Venipuncture (H3-A3). 1991. (Biology).
-
Antel J, Bania M, Noronha A, Neely S. Defective suppressor cell function mediated by T8+ cell lines from patients with progressive multiple sclerosis. J Immunol. 1986; 137:3436-3439. (Biology).
-
Bernard A, Boumsell L, Hill C. Joint report of the first international workshop on human leucocyte differentiation antigens by the investigators of the participating laboratories: T2 protocol. In: Bernard A. A. Bernard .. et al., ed. Leucocyte typing : human leucocyte differentiation antigens detected by monoclonal antibodies : specification, classification, nomenclature = Typage leucocytaire : antigènes de différenciation leucocytaire humains révélés par les anticorps monoclonaux : "Rapports des études communes". Berlin New York: Springer-Verlag; 1984:25-60.
-
Bishop G, Hall B, Duggin G, Horvath J, Sheil A, Tiller D. Immunopathology of renal allograft rejection analyzed with monoclonal antibodies to mononuclear cell markers. Kidney Internat. 1986; 29:708-717. (Biology).
-
Centers for Disease Control. Guidelines for the performance of CD4+ T-cell determination in persons with human immunodeficiency virus infection. MMWR. 1992; 41:1-17. (Biology).
-
Evans RL, Wall DW, Platsoucas CD, et al. Thymus-dependent membrane antigens in man: inhibition of cell-mediated lympholysis by monoclonal antibodies to the TH2 antigen. Proc Natl Acad Sci USA. 1981; 78:544-548. (Biology).
-
Gallagher PF, Fazekas de St. Groth B, Miller JFAP. CD4 and CD8 molecules can physically associate with the same T-cell receptor. Proc Natl Acad Sci USA. 1989; 86:10044-10048. (Biology).
-
Giorgi J, Hultin L. Lymphocyte subset alterations and immunophenotyping by flow cytometry in HIV disease. Clin Immunol Newslett. 1990; 10(4):55-61. (Biology).
-
Giorgi JV. Lymphocyte subset measurements: significance in clinical medicine. In: Rose NR, Friedman H, Fahey JL, ed. Manual of Clinical Laboratory Immunology. 3rd ed.. Washington, DC: American Society for Microbiology; 1986:236-246.
-
Gratama J, Naipal A, Oljans P, et al. T lymphocyte repopulation and differentiation after bone marrow transplantation: Early shifts in the ratio between T4+ and T8+ T lymphocytes correlate with the occurrence of acute graft-versus-host disease. Blood. 1984; 63(6):1416-1423. (Biology).
-
Jackson A. Basic phenotyping of lymphocytes: selection and testing of reagents and interpretation of data. Clin Immunol Newslett. 1990; 10:43-55. (Biology).
-
Jackson AL, Warner NL. Rose NR, Friedman H, Fahey JL, ed. Manual of Clincial Laboratory Immunology, Third Edition. Washington DC: American Society for Microbiology; 1986:226-235.
-
Lanier LL, Le AM, Phillips JH, Warner NL, Babcock GF. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983; 131(4):1789-1796. (Biology). View Reference
-
Ledbetter JA, Evans RL, Lipinski M, Cunningham-Rundles C, Good RA, Herzenberg LA. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981; 153(2):310-323. (Biology). View Reference
-
Mishell B, Shiigi S, Henry C, et al. Mishell B, Shiigi S, ed. Selected Methods in Cellular Immunology. New York: WH Freeman and Co; 1980:16-17.
-
National Committee for Clinical Laboratory Standards. Clinical Applications of Flow Cytometry: Quality Assurance and Immunophenotyping of Peripheral Blood Lymphocytes; Tentative Guideline. 1992; 1992. (Biology).
-
Prince H, Hirji K, Waldbeser L, Plaeger-Marshall S, Kleinman S, Lanier L. Influence of racial background on the distribution of T cell subsets and Leu-11-positive lymphocytes in healthy blood donors. Diag Immunol. 1985; 3:33-37. (Biology).
-
Reichert T, DeBruyere M, Deneys V, et al. Lymphocyte subset reference ranges in adult Caucasians. Clin Immunol Immunopathol. 1991; 60(2):190-208. (Biology). View Reference
-
Rudd CE, Burgess KE, Barber EK, Schlossman SF. Knapp W, Dörken B, Gilks WR, et al, ed. Leucocyte Typing IV: White Cell Differentiation Antigens. New York, NY: Oxford University Press; 1989:326-327.
-
Schmidt RE. Monoclonal antibodies for diagnosis of immunodeficiencies. Blut. 1989; 59:200-206. (Biology).
-
Wolde-Mariam W, Peter JB. Recent diagnostic advances in cellular immunology. Diag Med. 1984; 7:25-32. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For In Vitro Diagnostics Use.
Documents are subject to revision without notice. Please verify you have the correct revision of the document, and always refer back to BD's eIFU website for the latest and most up to date information.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.