-
Your selected location is
Middle East / Africa
- Change location/language
-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Transfusion
Blood transfusions are critical for saving lives. Timely transfusion of blood replenishes blood lost during surgical procedures or due to serious injury. Several conditions, such as hemophilia, sickle-cell anemia or cancer can also result in patients needing blood transfusions. At a global level, approximately 120 million units of blood are collected through blood donations every year.1 The donated blood is processed and tested before it is used for transfusion. While blood transfusion is generally safe, there are some risks, such as the inadvertent transmission or activation of viruses or development of serious allergic reactions, associated with it. We have made great strides in understanding the basis of these risks and have found ways to circumnavigate them.
What types of transfusion reactions can jeopardize the safety of blood transfusions?
Transfusions are generally safe and there are more successful red blood cell (RBC) transfusions than those that produce severe adverse effects.2 However, blood transfusions can induce immunosuppression by eliciting antibodies for human leukocyte antigen (HLA) or transmit viruses such as cytomegalovirus (CMV). Transfusion reactions, such as febrile non-hemolytic transfusion reaction (FNHTR) or platelet transfusion refractoriness, can be induced by leukocyte (or white blood cell, WBC) antibodies.3 The presence of WBCs in blood and platelet products is associated with an increased incidence of FNHTR, transmission of CMV and alloimmunization to HLA antigens in transfusion recipients.3 Depletion of leukocytes below a specific level can prevent HLA alloimmunization.4 Removal of >99.9% of leukocytes in platelets and red cell units can also reduce potential transmission of CMV.5
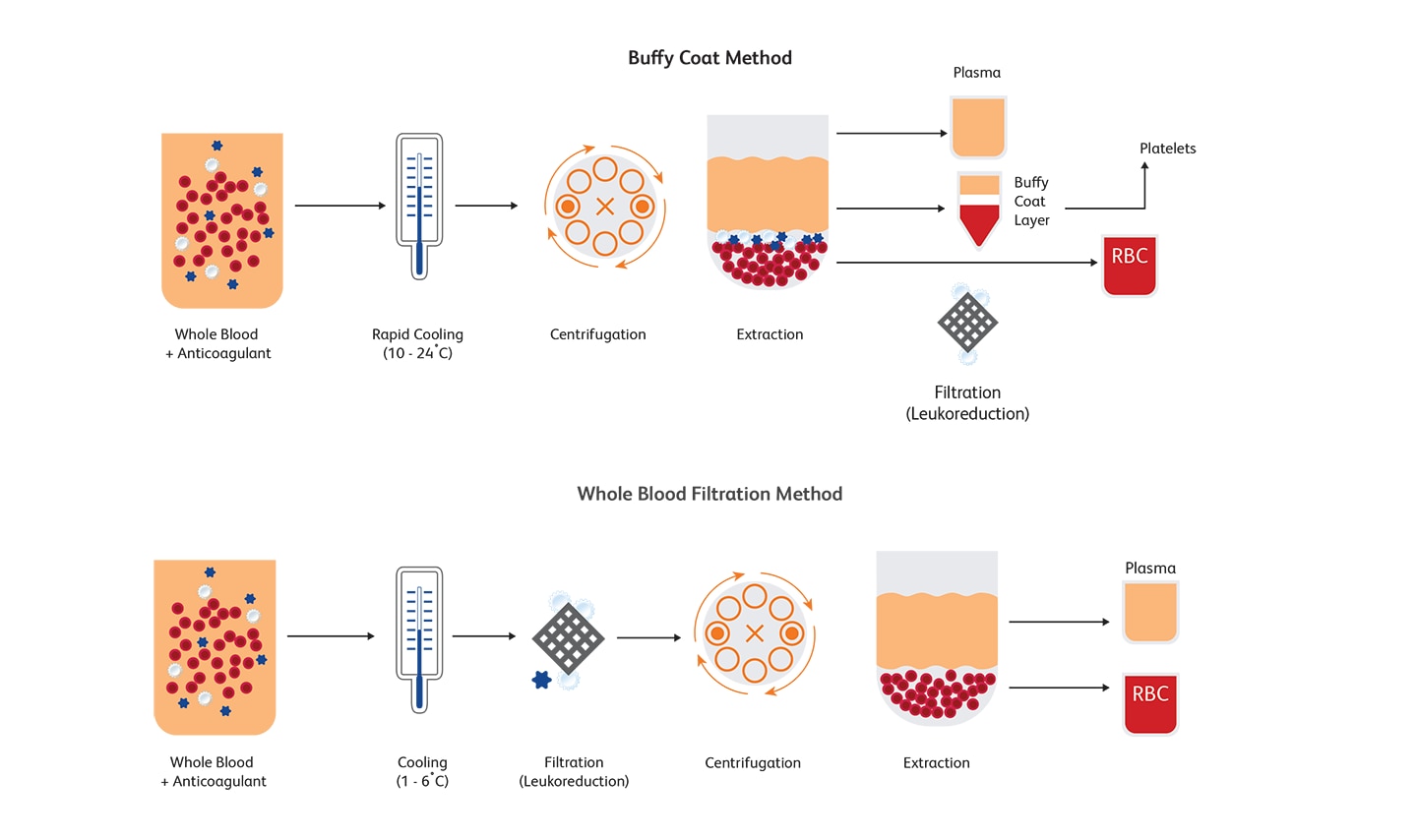
What is leukoreduction and how is it performed?
Leukoreduction (or leucoreduction) entails intentionally reducing the number of leukocytes from RBCs and platelets obtained from whole blood fractions or after apheresis. Centrifugation or filtration can reduce approximately 99.995% of leukocytes.6 Buffy coat method and whole blood filtration method are two commonly used approaches for reducing the number of leukocytes. Leukoreduction can lower the WBC count to 5 x 106 per unit or below, thus minimizing complications associated with transfusions.7,8 For more than three decades now, leukoreduction has become standard practice and is universally adopted to reduce adverse reactions resulting from blood transfusions.9 Guidelines for enforcing universal leukoreduction have been published to mitigate the risks associated with transfused blood products in the U.S., Canada and Europe; in the U.S., a WBC count of 5 x 106 per unit or below is acceptable before transfusion.
Flow cytometric evaluation of residual leukocytes
Enumerating residual leukocytes in leukoreduced blood products is critical to ensure the quality of blood components. Establishing reliable quality control processes is also equally important. Several methods to evaluate the number of residual leukocytes in blood after leukoreduction are available. Flow cytometry and counting cells using the manual Nageotte chamber or using automated leukocyte counters are well-established methods. Flow cytometry is an effective and FDA-cleared counting method for evaluating leukoreduced blood products.7 Comparisons evaluating the performance of different methods used for residual leukocyte enumeration show that flow cytometry is superior in terms of accuracy compared to Nageotte hemocytometry.8 Flow cytometry is also validated for accuracy, precision, linearity and robustness counting.10
The BD Leucocount™ Kit consists of the BD Leucocount™ Reagent (propidium iodide fluorescent dye) and BD Trucount™ Tubes and is intended for use with the BD FACSCalibur™ and BD FACSVia™ Flow Cytometer Systems or with a flow cytometer equipped with a 488-nm argon ion laser able to threshold on FL2, for enumerating residual white blood cells (rWBCs) in leukoreduced blood products.
References
- World Health Organization. https://www.who.int/health-topics/blood-transfusion-safety#tab=tab_1. Accessed September 29, 2020.
- Silliman CC, Burke T, Kelher MR. The accumulation of lipids and proteins during red blood cell storage: the roles of leukoreduction and experimental filtration. Blood Transfus. 2017;15(2):131-136. doi: 10.2450/2017.0314-16
- Bianchi M, Vaglio S, Pupella S, et al. Leucoreduction of blood components: an effective way to increase blood safety? Blood Transfus. 2016;14(3):214-227. doi: 10.2450/2015.0154-15
- Sniecinski I, O’Donnell MR, Hill LR. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988;71(5):1402-1407.
- de Graan-Hentzen YC, Gratama JW, Mudde GC, et al. Prevention of primary cytomegalovirus infection in patients with hematologic malignancies by intensive white cell depletion of blood products. Transfusion. 1989;29(9):757-760. doi: 10.1046/j.1537-2995.1989.29990070177.x
- Shapiro MJ. To filter blood or universal leukoreduction: what is the answer? Crit Care. 2004;8(Suppl 2):S27-S30. doi: 10.1186/cc2453
- Dumont LJ, Dzik WH, Rebulla P, Brandwein H. Practical guidelines for process validation and process control of white cell-reduced blood components: report of the Biomedical Excellence for Safer Transfusion (BEST) Working Party of the International Society of Blood Transfusion (ISBT). Transfusion. 1996;36(1):11-20. doi: 10.1046/j.1537-2995.1996.36196190510.x
- Dzik S, Moroff G, Dumont L. A multicenter study evaluating three methods for counting residual WBCs in WBC-reduced blood components: Nageotte hemocytometry, flow cytometry and microfluorometry. Transfusion. 2000;40(5):513-520. doi: 10.1046/j.1537-2995.2000.40050513.
- Silliman C, Burke T, K, Kelher MR. The accumulation of lips and proteins during red blood cell storage: the roles of leukoreduction and experimental filtration. Blood Transfus 2017;15:131-6. DOI 10.2450/2017.0314-16
- Categnaro S, Dragone P, Chieregato K, Alghisi A, Rodeghiero F, Astori G. Enumeration of residual white blood cells in leukoreduced blood products: Comparing flow cytometry with a portable microscopic cell counter. Transfus Apher Sci. 2016;54(2):266-270. doi: 10.1016/j.transci.2015.10.001
Interested in preparing for the IVDR? Visit our IVDR page to learn more about the measures you can take to prepare your lab.
BD Biosciences offers flow cytometers and assays for the enumeration of residual leukocytes in leukoreduced blood products.
The BD Leucocount™ Kit consists of the BD Leucocount™ Reagent and BD Trucount™ Tubes. The BD Leucocount™ Reagent contains propidium iodide (PI). PI is a nucleic acid dye that, when used with RNase, stains only cellular DNA. White blood cells are nucleated cells that contain DNA and are therefore stained with the dye. Nonnucleated particles (including platelets and red cells) do not stain with this reagent. BD Trucount™ Tubes contain beads that act as an internal reference to accurately determine the absolute count of residual white blood cells (rWBCs) in a single tube. Leukoreduced RBCs or platelets are combined with the lyophilized bead pellet in the BD Trucount™ Tubes before staining. After staining rWBCs, samples are acquired on a flow cytometer. Absolute rWBC counts are determined by using a simple calculation based on bead number and sample volume. The BD FACSVia™ and BD FACSCalibur™ Flow Cytometers were compared for accuracy and found to provide equivalent results of WBC absolute counts for leukoreduced RBCs and platelets.1
Sample data generated using the BD Leucocount™ Kit
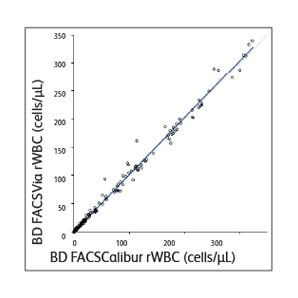
The BD Leucocount™ Kit consists of the BD Leucocount™ Reagent (propidium iodide fluorescent dye) and BD Trucount™ Tubes and is intended for use with the BD FACSCalibur™ and BD FACSVia™ Flow Cytometer Systems or with a flow cytometer equipped with a 488-nm argon ion laser able to threshold on FL2, for enumerating residual white blood cells (rWBCs) in leukoreduced blood products.
References
- Zeng Y, Dabay M, George V, et al. Comparison of flow cytometric methods for the enumeration of residual leucocytes in leucoreduced blood products: A multicenter study. Cytometry A. 2018;93(4):420-426. doi: 10.1002/cyto.a.23318
![]()
BD FACSVia™ System, BD Leucocount™ Kit and BD Trucount™ Tubes are in vitro diagnostic medical devices bearing a CE mark.
BD FACSCalibur™ Flow Cytometer and BD FACSVia™ System are discontinued.
BD Flow Cytometers are Class 1 Laser Products.