Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


.png)

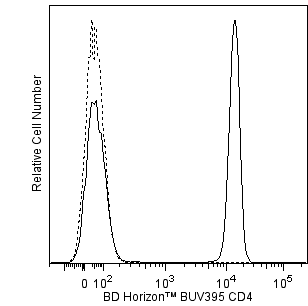
Two-color flow cytometric analysis of IL-13 expression in stimulated human lymphocytes. An enriched preparation of human peripheral blood CD4+ T cells was stimulated with immobilized Purified NA/LE Mouse Anti-Human CD3 (Cat. No. 555329; 10 μg/ml for plate coating) and soluble Purified NA/LE Mouse Anti-Human CD28 (Cat. No. 555725; 2 μg/ml) antibodies and Recombinant Human IL-2 (Cat. No. 554603; 10 ng/ml) and Human IL-4 (Cat. No. 554605; 25 ng/ml) proteins for 2 days. The stimulated cells were washed and cultured in medium containing IL-2 and IL-4 for another 3 days. Finally, the cells were harvested and restimulated for 5 hr with Phorbol 12-Myristate 13-Acetate (PMA; Sigma, Cat. No. P-8139; 50 ng/ml) and Ionomycin (Sigma, Cat. No. I-0634; 1 μg/ml) in the presence of BD GolgiStop™ Protein Transport Inhibitor (containing Monensin) (Cat. No. 554724). The cells were harvested and washed with BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) and fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655). The cells were then washed and stained in BD Perm/Wash™ Buffer (Cat. No. 554723) with BD Horizon™ BUV395 Mouse Anti-Human CD4 antibody (Cat No. 564724) and with either BD Horizon™ R718 Rat IgG1 κ Isotype Control (Cat. No. 566948; Left Plot) or BD Horizon™ R718 Mouse Anti-Human IL-13 antibody (Cat. No. 567094/567247; Right Plot) using BD Biosciences Intracellular Cytokine Staining protocol. A pseudocolor density plot showing the correlated expression of IL-13 (or Ig Isotype control staining) versus CD4 was derived from gated events with the forward and side light-scatter characteristics of intact stimulated lymphocytes. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ software.
.png)

BD Horizon™ R718 Rat Anti-Human IL-13
.png)
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD™ CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to BD CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD CompBead to ensure that BD CompBeads are appropriate for your specific cellular application.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- This product is provided under an Agreement between BIOTIUM and BD Biosciences. This product, and only in the amount purchased by buyer, may be used solely for buyer’s own internal research, in a manner consistent with the accompanying product literature. No other right to use, sell or otherwise transfer (a) this product, or (b) its components is hereby granted expressly, by implication or by estoppel. This product is for research use only. Diagnostic uses require a separate license from Biotium, Inc. For information on purchasing a license to this product including for purposes other than research, contact Biotium, Inc., 3159 Corporate Place, Hayward, CA 94545, Tel: (510) 265-1027. Fax: (510) 265-1352. Email: btinfo@biotium.com.
- Alexa Fluor™ is a trademark of Life Technologies Corporation.
Companion Products





The JES10-5A2 monoclonal antibody specifically binds to human interleukin-13, IL-13. IL-13 is produced by activated T cells, mast cells and NK cells. IL-13 regulates IgE production by B cells and can suppress the cytotoxic activity of macrophages and their production of inflammatory mediators. The immunogen used to produce the JES10-5A2 hybridoma was COS-expressed recombinant human IL-13. This is a neutralizing antibody.
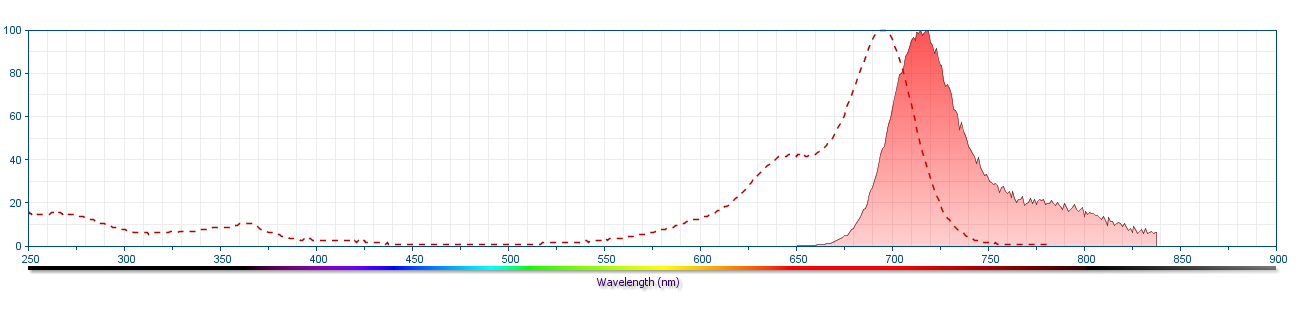
The antibody was conjugated to BD Horizon Red 718, which has been developed exclusively for BD Biosciences as a better alternative to Alexa Fluor® 700. BD Horizon Red 718 can be excited by the red laser (628 – 640 nm) and, with an Em Max around 718 nm, it can be detected using a 730/45 nm filter. Due to similar excitation and emission properties, we do not recommend using R718 in combination with APC-R700 or Alexa Fluor® 700.
Development References (7)
-
Abrams J. Immunoenzymetric assay of mouse and human cytokines using NIP-labeled anti-cytokine antibodies. Curr Protoc Immunol. 2001; 1:6.20-6.21. (Clone-specific). View Reference
-
Andersson J, Abrams J, Bjork L, et al. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994; 83(1):16-24. (Clone-specific: Immunohistochemistry). View Reference
-
Andersson U, Andersson J. Immunolabeling of cytokine-producing cells in tissues and in suspension. In: Fradelizie D, Emelie D, ed. Cytokine Producing Cells. Paris: Inserm; 1994:32-49.
-
Litton M, Andersson J, Bjork L, Fehniger T, Ulfgren AK, Andersson U. Cytoplasmic cytokine staining in individual cells. In: Debets and Savelkoul, ed. Human Cytokine Protocols. Humana Press; 1996.
-
McKenzie ANJ, Matthews DJ. IL-13. In: Oppenheim JJ, Feldmann M, Durum SK, Hirano T, Vilcek J, Nicola NA, ed. Cytokine Reference : A compendium of cytokines and other mediators of host defense. San Diego: Academic Press; 2001:203-211.
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology: Flow cytometry). View Reference
-
Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000; 192(11):1553-1562. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.