-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Dehydrated Culture Media
-
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- Canada (English)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
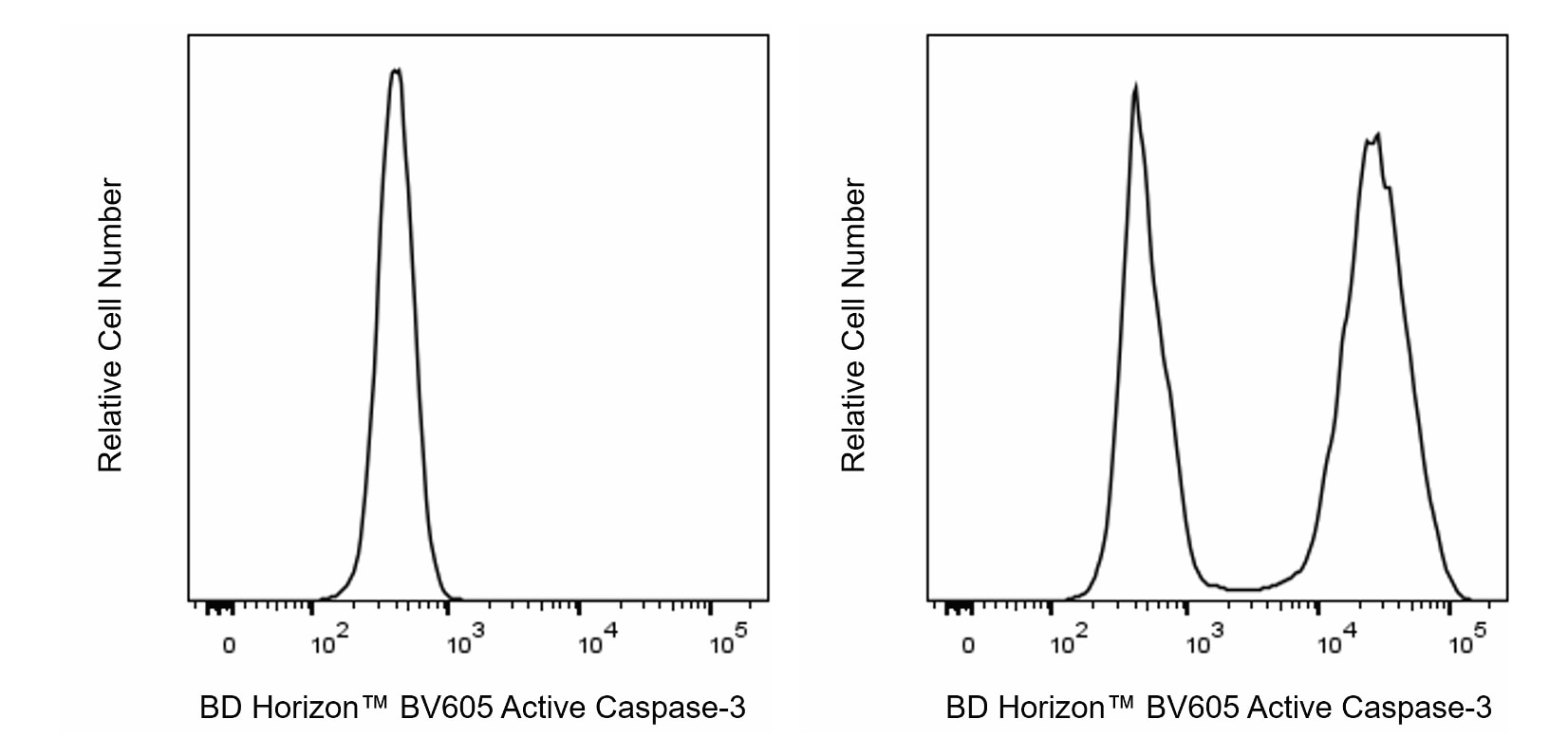




Flow cytometric analysis of Active Caspase-3 expression by Apoptotic and Non-apoptotic Human Jurkat cells. Cells from the Human Jurkat (T-cell leukemia, ATCC® TIB-152™) cell line were left untreated (Left histogram) or treated with 12 μM of Camptothecin for 6 hours to induce apoptosis (Right histogram). Cells were washed, fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655) and then permeabilized with BD Perm/Wash™ Buffer (Cat. No. 554723). The cells were subsequently stained in BD Perm/Wash™ Buffer with BD Horizon™ BV605 Rabbit Anti-Active Caspase-3 antibody (Cat. No. 570538/570539) at 0.5 µg/test. The fluorescence histograms showing Active Caspase-3 expression were derived from gated events with the forward and side light-scatter characteristics of single cells. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ Software. Data shown on this Technical Data Sheet are not lot specific.

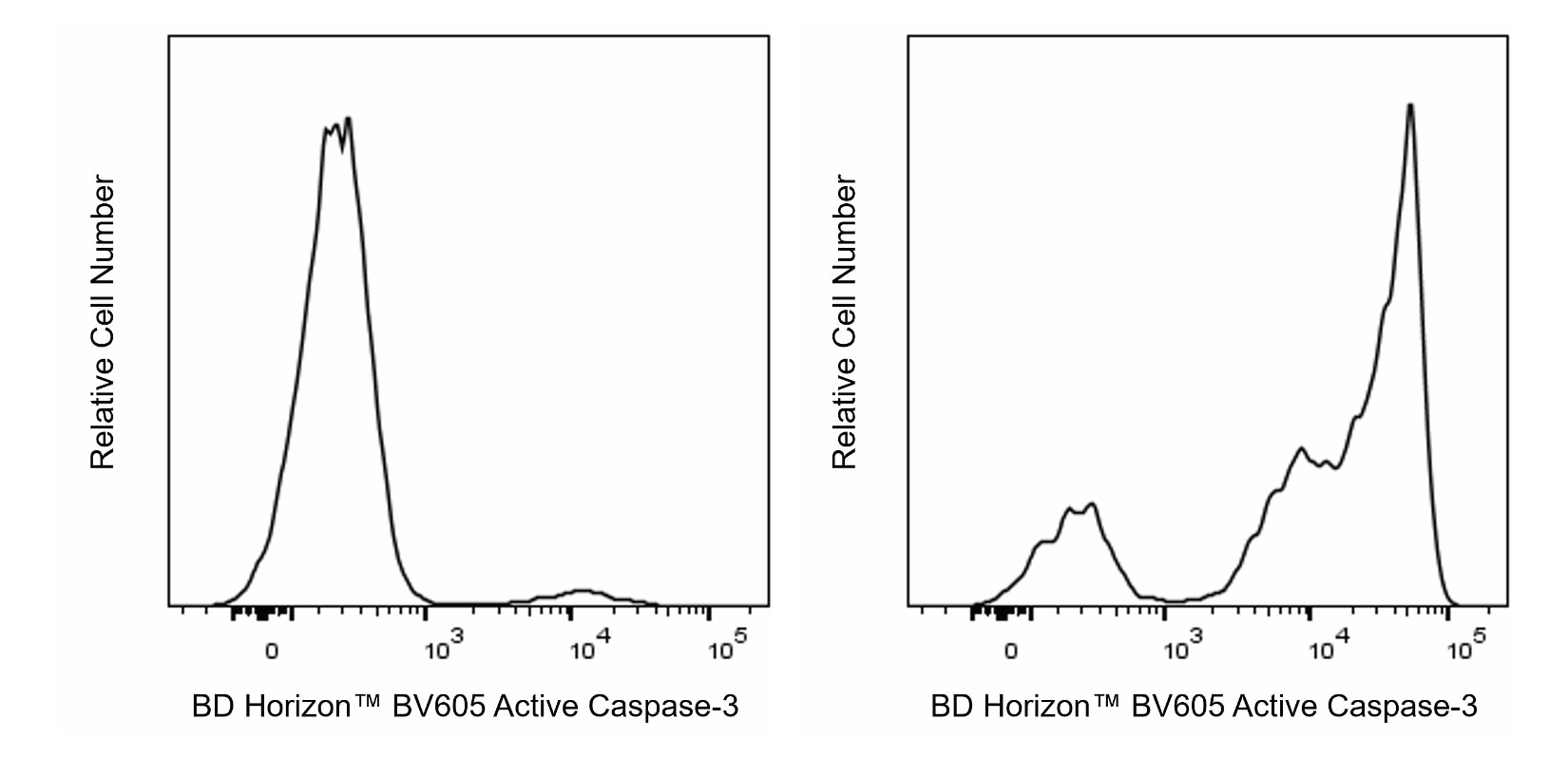
Flow cytometric analysis of Active Caspase-3 expression by Apoptotic and Non-apoptotic Mouse thymocytes. C57BL/6 Mouse thymocytes were left untreated (Left histogram) or treated with 1 μM of Dexamethasone for 5 hours to induce apoptosis (Right histogram). Cells were washed, fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655) and then permeabilized with BD Perm/Wash™ Buffer (Cat. No. 554723). The cells were subsequently stained in BD Perm/Wash™ Buffer with BD Horizon™ BV605 Rabbit Anti-Active Caspase-3 antibody (Cat. No. 570538/570539) at 0.5 µg/test. The fluorescence histograms showing Active Caspase-3 expression were derived from gated events with the forward and side light-scatter characteristics of single cells. Flow cytometry and data analysis were performed using a BD LSRFortessa™ X-20 Cell Analyzer System and FlowJo™ Software. Data shown on this Technical Data Sheet are not lot specific.


BD Horizon™ BV605 Rabbit Anti-Active Caspase-3

BD Horizon™ BV605 Rabbit Anti-Active Caspase-3

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD® CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
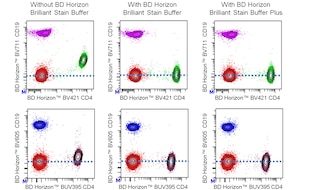
For optimal and reproducible results, BD Horizon Brilliant™ Stain Buffer should be used anytime BD Horizon Brilliant dyes are used in a multicolor flow cytometry panel. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. When BD Horizon Brilliant Stain Buffer is used in in the multicolor panel, it should also be used in the corresponding compensation controls for all dyes to achieve the most accurate compensation. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Please observe the following precautions: We recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to protect exposure of conjugated reagents, including cells stained with those reagents, to any room illumination. Absorption of visible light can significantly affect the emission spectra and quantum yield of tandem fluorochrome conjugates.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Although every effort is made to minimize the lot-to-lot variation in the efficiency of the fluorochrome energy transfer, differences in the residual emission from BD Horizon™ BV421 may be observed. Therefore, we recommend that individual compensation controls be performed for every BD Horizon™ BV605 conjugate.
- Human donor specific background has been observed in relation to the presence of anti-polyethylene glycol (PEG) antibodies, developed as a result of certain vaccines containing PEG, including some COVID-19 vaccines. We recommend use of BD Horizon Brilliant™ Stain Buffer in your experiments to help mitigate potential background. For more information visit https://www.bdbiosciences.com/en-us/support/product-notices.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- For U.S. patents that may apply, see bd.com/patents.
Companion Products





The C92-605.rMAb is a recombinant monoclonal antibody derived from C92-605 hybridoma cells. This antibody specifically recognizes the active form of Caspase-3 in human and mouse cells. It has not been reported to recognize the pro-enzyme form of Caspase-3. The Caspase family of cysteine proteases play crucial roles in apoptosis and inflammation. Caspase-3 is a key protease that is activated during the early stages of apoptosis and, like other members of the Caspase family, is synthesized as an inactive pro-enzyme that is processed in cells undergoing apoptosis by self-proteolysis and/or cleavage by another protease. The processed forms of caspases consist of large (17-22 kDa) and small (10-12 kDa) subunits which associate to form an active enzyme. Active Caspase-3, a marker for cells undergoing apoptosis, consists of a heterodimer of 17 and 12 kDa subunits which is derived from the 32 kDa pro-enzyme. Active Caspase-3 proteolytically cleaves and activates other caspases, as well as relevant targets in the cytoplasm, eg, D4-GDI and Bcl-2, and in the nucleus (eg, PARP).
Development References (5)
-
Dukers DF, Oudejans JJ, Vos W, ten Berge RL, Meijer CJ. Apoptosis in B-cell lymphomas and reactive lymphoid tissues always involves activation of caspase 3 as determined by a new in situ detection method. J Pathol. 2002; 196(3):307-315. (Clone-specific: Immunohistochemistry, Immunoprecipitation). View Reference
-
Ohsawa S, Hamada S, Yoshida H, Miura M. Caspase-mediated changes in histone H1 in early apoptosis: prolonged caspase activation in developing olfactory sensory neurons. Cell Death Differ. 2008; 15(9):1429-1439. (Clone-specific: Fluorescence microscopy, Immunofluorescence, Western blot). View Reference
-
Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO. CD99 signals caspase-independent T cell death. J Immunol. 2001; 166(8):4931-4942. (Clone-specific: Intracellular Staining/Flow Cytometry). View Reference
-
Winzler C, Fantinato M, Giordan M, Calore E, Basso G, Messina C. CD4(+) T regulatory cells are more resistant to DNA damage compared to CD4(+) T effector cells as revealed by flow cytometric analysis.. Cytometry A. 2011; 79(11):903-11. (Clone-specific: Intracellular Staining/Flow Cytometry). View Reference
-
Zhu S, Zhang X, Weichert-Leahey N, et al. LMO1 Synergizes with MYCN to Promote Neuroblastoma Initiation and Metastasis.. Cancer Cell. 2017; 32(3):310-323.e5. (Clone-specific: Immunohistochemistry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.