-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Dehydrated Culture Media
-
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- Canada (English)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes (including BD OptiBuild Brilliant reagents) are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794).
Product Notices
- This antibody was developed for use in flow cytometry.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Ultraviolet 661 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,575,303; 8,354,239.
Companion Products
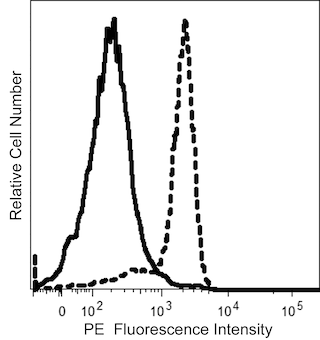



The GA-R2 (also known as HIR2) monoclonal antibody specifically binds to CD235a and CD235b. CD235a is also known as Glycophorin A (GYPA, GPA, GLPA), Sialoglycoprotein alpha, MN sialoglycoprotein, or PAS-2. CD235b is otherwise known as Glycophorin B (GYPB, GPB, GLPB), Sialoglycoprotein delta, SS-active sialoglycoprotein, or PAS-3. CD235a and CD235b are type I transmembrane sialoglycoproteins that are expressed on human erythrocytes, erythroid precursor cells and certain leukemic cell types. CD235a carries blood group M and N antigens, whereas CD235b contains S, s, and U antigens. This antibody is useful for the identification and characterization of erythrocytes, certain myeloid leukemic cell types, and studies of erythroid cell development and infectious diseases with erythrocyte involvement. Glycophorins may play a role in preventing cell agglutination.
The antibody was conjugated to BD Horizon™ BUV661 which is part of the BD Horizon Brilliant™ Ultraviolet family of dyes. This dye is a tandem fluorochrome of BD Horizon BUV395 with an Ex Max of 348-nm and an acceptor dye with an Em Max at 661-nm. BD Horizon Brilliant BUV661 can be excited by the ultraviolet laser (355 nm) and detected with a 670/25 filter and a 630 nm LP. Due to cross laser excitation of this dye, there may be significant spillover into channels detecting APC-like emissions (eg, 670/25-nm filter).
Due to spectral differences between labeled cells and beads, using BD™ CompBeads can result in incorrect spillover values when used with BD Horizon BUV661 reagents. Therefore, the use of BD CompBeads or BD CompBeads Plus to determine spillover values for these reagents is not recommended. Different BUV661 reagents (eg, CD4 vs. CD45) can have slightly different fluorescence spillover therefore, it may also be necessary to use clone-specific compensation controls when using these reagents.
Development References (7)
-
Bain BJ. Leukemia diagnosis: A guide to the FAB classification. 1990.
-
Blanchard D, Roux YP-L, Vusio P, Follea G. Characterization of monoclonal antibodies directed to human red blood cell glycophorins A and B. In: Mason D. David Mason .. et al., ed. Leucocyte typing VII : white cell differentiation antigens : proceedings of the Seventh International Workshop and Conference held in Harrogate, United Kingdom. Oxford: Oxford University Press; 2002:579-582.
-
Gross S, Helm K, Gruntmeir JJ, Stillman WS, Pyatt DW, Irons RD. Characterization and phenotypic analysis of differentiating CD34+ human bone marrow cells in liquid culture. Br J Haematol. 1997; 5(318):326. (Clone-specific: Flow cytometry). View Reference
-
Keren DF, Hanson CA, Hurtubise PE. David F. Keren, Curtis A. Hanson, Paul E. Hurtubise., ed. Flow cytometry and clinical diagnosis. Chicago: ASCP Press; 1994:1-676.
-
Loken MR, Civin CI, Bigbee WL, Langlois RG, Jensen RH. Coordinate glycosylation and cell surface expression of glycophorin A during normal human erythropoiesis. Blood. 1987; 70(6):1959-1961. (Biology). View Reference
-
Nakahata T, Okumura N. Cell surface antigen expression in human erythroid progenitors: erythroid and megakaryocytic markers. Leuk Lymphoma. 1994; 13(5-6):401-409. (Biology). View Reference
-
Rogers CE, Bradley MS, Palsson BO, Koller MR. Flow cytometric analysis of human bone marrow perfusion cultures: erythroid development and relationship with burst-forming units-erythroid. Exp Hematol. 1996; 24(5):597-604. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.