-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Dehydrated Culture Media
-
- BD® AbSeq Assay
- BD Rhapsody™ Accessory Kits
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-Guard Sample Preservation Buffer
- BD Rhapsody™ ATAC-Seq Assays
- Canada (English)
-
Change country/language
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


PE Mouse Anti-Human CD103
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Store vials at 2°C to 8°C. Conjugated forms should not be frozen. Protect from exposure to light. Each reagent is stable until the expiration date shown on the bottle label when stored as directed.
The CD103 antibody, clone Ber-ACT8, is derived using standard cell hybridization procedures using spleen cells from BALB/c mice immunized with the HTLV-1–positive T-cell line, MAPS16. The CD103 antibody recognizes the αE subunit of integrin αEβ
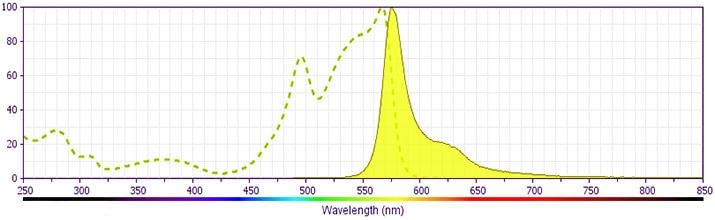
Development References (14)
-
Brew R, West DC, Burthem J, Christmas SE. Expression of the human mucosal lymphocyte antigen, HML-1, by T cells activated with mitogen or specific antigen in vitro. Scan J Immunol. 1995; 41:553–562. (Biology).
-
Centers for Disease Control. Update: universal precautions for prevention of transmission of human immunodeficiency virus, hepatitis B virus, and other bloodborne pathogens in healthcare settings. MMWR. 1988; 37:377-388. (Biology).
-
Cerf-Benussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987; 17:1279–1285. (Biology).
-
Clinical and Laboratory Standards Institute. 2005. (Biology).
-
Falini B, Pileri SA, Flenghi L, et al. Selection of a panel of monoclonal antibodies for monitoring residual disease in peripheral blood and bone marrow of interferon-treated hairy cell leukaemia patients. Br J Haematol. 1990; 76:460–468. (Biology).
-
Hadley GA, Rostapshova EA, Gomolka DM, et al. Regulation of the epithelial cell-specific integrin, CD103, by human CD8+ cytolytic T lymphocytes. Transplantation. 1999; 67:1418–1425. (Biology).
-
Hassan IB, Hagberg H, Sundström C. Immunophenotype of hairy-cell leukemia. Eur J Haematol. 1990; 45:172-176. (Biology).
-
Kruschwitz M, Fritzsche G, Schwarting R, et al. Ber-ACT8: new monoclonal antibody to the mucosa lymphocyte antigen. J Clin Pathol. 1991; 44(8):636-645. (Biology). View Reference
-
Lohmeyer J, Friedrish J, Grimminger F, et al. Expression of mucosa-related integrin αEβ7 on alveolar T cells in interstitial lung diseases. Clin Exp Immunol. 1999; 116:340-346. (Biology).
-
Micklem KJ, Dong Y, Willis A, et al. HML-1 antigen on mucosa-associated T cells, activated cells, and hairy leukemic cells is a new integrin containing the β7 subunit. Am J Pathol. 1996; 3:30-36. (Biology).
-
Moldenhauer G, Mielke B, Dörken B, Schwartz-Albiez R, Möller P. Identity of HML-1 antigen on intestinal intraepithelial T cells and of B-ly7 antigen on hairy cell leukaemia. Scand J Immunol. 1990; 32:77–82. (Biology).
-
Möller P, Mielke B, Moldenhauer G. Monoclonal antibody HML-1, a marker for intraepithelial T cells and lymphomas derived thereof, also recognizes hairy cell leukemia and some B-cell lymphomas. Am J Pathol. 1990; 136:509–512. (Biology).
-
Parker CM, Cepek KL, Russell GJ, et al. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992; 89(5):1924-1928. (Biology). View Reference
-
Rihs S, Walker C, Virchow JC Jr, et al. Differential expression of α E β 7 integrins on bronchoalveolar lavage T lymphocyte subsets: regulation by α 4 β 1-integrin crosslinking and TGF-β. Am J Respir Cell Mol Biol. 1996; 15:600–610. (Biology).
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Although not required, these products are manufactured in accordance with Good Manufacturing Practices.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.