-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Dehydrated Culture Media
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Canada (English)
-
Change country/language
Old Browser
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer

Figure 1. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining of leukocytes. Human leukocytes were stained with either MOPC-21 or X-40 Mouse IgG1, κ Isotype Controls (mIgG1, κ Iso Ctrl) conjugated with either APC-Cy7, APC-H7, PE-Cy5, PE-Cy7, or PE-CF594. These controls were either not premixed (Upper Plots) or premixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer as indicated. The cells were washed and analyzed. Bivariate pseudocolor density plots show reduced background staining on leukocyte that were stained in the presence of this buffer. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.
Figure 2. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of human leukocytes with PE-Cy7 antibodies. PE-Cy7-conjugated clones specific for human CD14 (M5E2), CD33 (P67.6), CD64 (10.1), CD279/PD-1 (EH12.1), CD3 (UCHT1), CD19 (HIB19) either were not mixed (Upper Plots) or were mixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer. After staining leukocytes with these, they were washed and analyzed. The plots show reduced background staining on leukocytes that were stained using this buffer.
Figure 3. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of mouse bone marrow cells (BMC) for CD11b. Mouse BMC were either not preincubated (Top Plots) or were preincubated (Middle, Bottom Plots) with BD Pharmingen™ Mouse BD Fc Block™ as indicated. PE-Cy7 Rat IgG2b, κ Isotype Control (Left Plots) or PE-Cy7 Rat Anti-CD11b (Right Plots) were either not premixed (Top and Middle Plots) or were mixed (Bottom Plots) with this buffer. The cells were then stained with the PE-Cy7- reagents, washed, and similarly analyzed by flow cytometry.

Figure 1. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining of leukocytes. Human leukocytes were stained with either MOPC-21 or X-40 Mouse IgG1, κ Isotype Controls (mIgG1, κ Iso Ctrl) conjugated with either APC-Cy7, APC-H7, PE-Cy5, PE-Cy7, or PE-CF594. These controls were either not premixed (Upper Plots) or premixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer as indicated. The cells were washed and analyzed. Bivariate pseudocolor density plots show reduced background staining on leukocyte that were stained in the presence of this buffer. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.
Figure 2. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of human leukocytes with PE-Cy7 antibodies. PE-Cy7-conjugated clones specific for human CD14 (M5E2), CD33 (P67.6), CD64 (10.1), CD279/PD-1 (EH12.1), CD3 (UCHT1), CD19 (HIB19) either were not mixed (Upper Plots) or were mixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer. After staining leukocytes with these, they were washed and analyzed. The plots show reduced background staining on leukocytes that were stained using this buffer.
Figure 3. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of mouse bone marrow cells (BMC) for CD11b. Mouse BMC were either not preincubated (Top Plots) or were preincubated (Middle, Bottom Plots) with BD Pharmingen™ Mouse BD Fc Block™ as indicated. PE-Cy7 Rat IgG2b, κ Isotype Control (Left Plots) or PE-Cy7 Rat Anti-CD11b (Right Plots) were either not premixed (Top and Middle Plots) or were mixed (Bottom Plots) with this buffer. The cells were then stained with the PE-Cy7- reagents, washed, and similarly analyzed by flow cytometry.


Figure 1. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining of leukocytes. Human leukocytes were stained with either MOPC-21 or X-40 Mouse IgG1, κ Isotype Controls (mIgG1, κ Iso Ctrl) conjugated with either APC-Cy7, APC-H7, PE-Cy5, PE-Cy7, or PE-CF594. These controls were either not premixed (Upper Plots) or premixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer as indicated. The cells were washed and analyzed. Bivariate pseudocolor density plots show reduced background staining on leukocyte that were stained in the presence of this buffer. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.
Figure 2. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of human leukocytes with PE-Cy7 antibodies. PE-Cy7-conjugated clones specific for human CD14 (M5E2), CD33 (P67.6), CD64 (10.1), CD279/PD-1 (EH12.1), CD3 (UCHT1), CD19 (HIB19) either were not mixed (Upper Plots) or were mixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer. After staining leukocytes with these, they were washed and analyzed. The plots show reduced background staining on leukocytes that were stained using this buffer.
Figure 3. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of mouse bone marrow cells (BMC) for CD11b. Mouse BMC were either not preincubated (Top Plots) or were preincubated (Middle, Bottom Plots) with BD Pharmingen™ Mouse BD Fc Block™ as indicated. PE-Cy7 Rat IgG2b, κ Isotype Control (Left Plots) or PE-Cy7 Rat Anti-CD11b (Right Plots) were either not premixed (Top and Middle Plots) or were mixed (Bottom Plots) with this buffer. The cells were then stained with the PE-Cy7- reagents, washed, and similarly analyzed by flow cytometry.
Figure 1. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining of leukocytes. Human leukocytes were stained with either MOPC-21 or X-40 Mouse IgG1, κ Isotype Controls (mIgG1, κ Iso Ctrl) conjugated with either APC-Cy7, APC-H7, PE-Cy5, PE-Cy7, or PE-CF594. These controls were either not premixed (Upper Plots) or premixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer as indicated. The cells were washed and analyzed. Bivariate pseudocolor density plots show reduced background staining on leukocyte that were stained in the presence of this buffer. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.
Figure 2. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of human leukocytes with PE-Cy7 antibodies. PE-Cy7-conjugated clones specific for human CD14 (M5E2), CD33 (P67.6), CD64 (10.1), CD279/PD-1 (EH12.1), CD3 (UCHT1), CD19 (HIB19) either were not mixed (Upper Plots) or were mixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer. After staining leukocytes with these, they were washed and analyzed. The plots show reduced background staining on leukocytes that were stained using this buffer.
Figure 3. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of mouse bone marrow cells (BMC) for CD11b. Mouse BMC were either not preincubated (Top Plots) or were preincubated (Middle, Bottom Plots) with BD Pharmingen™ Mouse BD Fc Block™ as indicated. PE-Cy7 Rat IgG2b, κ Isotype Control (Left Plots) or PE-Cy7 Rat Anti-CD11b (Right Plots) were either not premixed (Top and Middle Plots) or were mixed (Bottom Plots) with this buffer. The cells were then stained with the PE-Cy7- reagents, washed, and similarly analyzed by flow cytometry.

Figure 1. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining of leukocytes. Human leukocytes were stained with either MOPC-21 or X-40 Mouse IgG1, κ Isotype Controls (mIgG1, κ Iso Ctrl) conjugated with either APC-Cy7, APC-H7, PE-Cy5, PE-Cy7, or PE-CF594. These controls were either not premixed (Upper Plots) or premixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer as indicated. The cells were washed and analyzed. Bivariate pseudocolor density plots show reduced background staining on leukocyte that were stained in the presence of this buffer. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.
Figure 2. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of human leukocytes with PE-Cy7 antibodies. PE-Cy7-conjugated clones specific for human CD14 (M5E2), CD33 (P67.6), CD64 (10.1), CD279/PD-1 (EH12.1), CD3 (UCHT1), CD19 (HIB19) either were not mixed (Upper Plots) or were mixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer. After staining leukocytes with these, they were washed and analyzed. The plots show reduced background staining on leukocytes that were stained using this buffer.
Figure 3. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of mouse bone marrow cells (BMC) for CD11b. Mouse BMC were either not preincubated (Top Plots) or were preincubated (Middle, Bottom Plots) with BD Pharmingen™ Mouse BD Fc Block™ as indicated. PE-Cy7 Rat IgG2b, κ Isotype Control (Left Plots) or PE-Cy7 Rat Anti-CD11b (Right Plots) were either not premixed (Top and Middle Plots) or were mixed (Bottom Plots) with this buffer. The cells were then stained with the PE-Cy7- reagents, washed, and similarly analyzed by flow cytometry.

Figure 1. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining of leukocytes. Human leukocytes were stained with either MOPC-21 or X-40 Mouse IgG1, κ Isotype Controls (mIgG1, κ Iso Ctrl) conjugated with either APC-Cy7, APC-H7, PE-Cy5, PE-Cy7, or PE-CF594. These controls were either not premixed (Upper Plots) or premixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer as indicated. The cells were washed and analyzed. Bivariate pseudocolor density plots show reduced background staining on leukocyte that were stained in the presence of this buffer. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.
Figure 2. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of human leukocytes with PE-Cy7 antibodies. PE-Cy7-conjugated clones specific for human CD14 (M5E2), CD33 (P67.6), CD64 (10.1), CD279/PD-1 (EH12.1), CD3 (UCHT1), CD19 (HIB19) either were not mixed (Upper Plots) or were mixed (Lower Plots) with BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer. After staining leukocytes with these, they were washed and analyzed. The plots show reduced background staining on leukocytes that were stained using this buffer.
Figure 3. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces nonspecific staining but preserves specific staining of mouse bone marrow cells (BMC) for CD11b. Mouse BMC were either not preincubated (Top Plots) or were preincubated (Middle, Bottom Plots) with BD Pharmingen™ Mouse BD Fc Block™ as indicated. PE-Cy7 Rat IgG2b, κ Isotype Control (Left Plots) or PE-Cy7 Rat Anti-CD11b (Right Plots) were either not premixed (Top and Middle Plots) or were mixed (Bottom Plots) with this buffer. The cells were then stained with the PE-Cy7- reagents, washed, and similarly analyzed by flow cytometry.

BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer

BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For more information on MonoBlock™ Leukocyte Staining Buffer, please visit our website at: https://www.bdbiosciences.com/monoblock
Protocols for Multicolor Immunofluorescent Surface Staining of Cells using BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer
Protocol 1 for Staining Peripheral Blood Mononuclear Cells (PBMC) or Bulk Erythrocyte-lysed Whole Blood or Cell Samples Prepared from Lymphoid Tissues.
a. First add 10 μL BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer to each staining tube or plate microwell followed by addition of fluorescent antibody or antibody cocktails and carefully vortex.
b. Add 100 μL containing suspended human or mouse leukocytes (eg, at ~10^6 cells) to each tube or microwell and carefully vortex.
Alternatively: Can first add 10 μL of BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer and 100 μL containing suspended leukocytes to each tube or microwell and carefully vortex. Then add fluorescent antibody or antibody cocktail to the mixture of cells and blocking buffer.
c. Incubate the suspended cells (30 min) protected from light at room temperature (RT) for human cells and at 4°C for mouse cells.
d. After incubation, wash the cells twice with BD Pharmingen™ Stain Buffer (FBS) [Cat. No. 554656].
e. Resuspend cells in 500 μL BD Pharmingen™ Stain Buffer (FBS) for flow cytometric analysis.
Protocol 2 for Staining Human Whole Blood
a. First add 10 μL BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer to each staining tube followed by addition of fluorescent antibody or antibody cocktails and carefully vortex.
b. Add 100 μL of human whole blood.
Alternatively: Can first add 10 μL of BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer and 100 μL containing suspended human whole blood to each tube or microwell and carefully vortex. Then add fluorescent antibody or antibody cocktail to the mixture of cells and blocking buffer.
c. Incubate the suspended cells (30 min) at room temperature (RT) protected from light.
d. After incubation, lyse erythrocytes with BD Pharm Lyse™ Lysing Buffer (Cat. No. 555899) or BD FACS™ Lysing Solution (Cat. No. 349202).
e. Wash the cells twice with BD Pharmingen™ Stain Buffer (FBS) [Cat. No. 554656].
f. Resuspend cells in 500 μL BD Pharmingen™ Stain Buffer (FBS) for flow cytometric analysis.
Protocol Notes:
• For human leukocytes, further reduction in potential nonspecific staining caused by fluorescent antibody binding to cellular Fc receptors can be reduced by preincubating the cells with BD Pharmingen™ Human BD Fc Block™ (Cat. No. 564219/564220).
• For mouse leukocytes, further reduction in potential nonspecific staining caused by fluorescent antibody binding to cellular Fc receptors can similarly be reduced by preincubating the cells with BD Pharmingen™ Purified Rat Anti-Mouse CD16/CD32 antibody (Mouse BD Fc Block™) [Cat. No. 553141/553142].
• BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer is compatible with BD Horizon™ Brilliant Stain Buffer (Cat. No. 566349 563749) and BD Horizon™ Brilliant Stain Buffer plus (Cat. No. 566385 568264). Data not shown but available upon request.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- CF™ is a trademark of Biotium, Inc.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
- For U.S. patents that may apply, see bd.com/patents.
Data Sheets
Companion Products



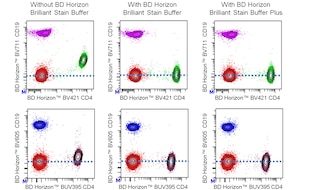

Although the exact mechanism is not clear, some cyanine-type dyes (PE-Cy5, PerCP-Cy5.5, PE-Cy7, APC-Cy7, APC-H7, and others) and non-cyanine tandem dyes (eg, PE-CF594) are known to bind to human and mouse monocytes, macrophages, and other leukocyte subsets. BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer reduces this nonspecific background staining, while not impacting specific surface staining or cell viability. This buffer is added directly to leukocytes or added to fluorescent reagent cocktails prior to staining leukocytes. This reagent may be complemented by others including i) BD Horizon™ Brilliant Stain Buffer, which mitigates dye-dye interactions amongst BD Horizon™ Brilliant fluorochromes, and ii) BD Fc Block™ reagents. The latter can reduce potential nonspecific staining caused by fluorescent antibodies that bind to Fc receptors expressed by human and mouse cells. For optimal and reproducible surface staining results, BD Pharmingen™ MonoBlock™ Leukocyte Staining Buffer should be used with cyanine-like and other tandem dye-conjugated reagents that can potentially stain cells nonspecifically.
No Citations Are Available for this Product
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.