-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Cancer Biology
Characteristics, such as sustained proliferation, circumventing growth suppression, evading destruction by immune cells, enabling replicative immortality, inducing angiogenesis and resisting cell death are considered hallmarks of cancer.1 The enhancement of tumorigenesis by oncogenes and release of tumor growth inhibition by tumor suppressor genes together contribute toward cancer progression. In addition, reprogramming of cellular metabolism, caused by alterations in intracellular and extracellular metabolites, can also influence the tumor microenvironment (TME), resulting in tumorigenesis.2
Types of Cancers
Cancer classification is based on the embryological origin of the tissue from which the cancer develops. Types of cancers include carcinoma, sarcoma, hematopoietic (e.g., leukemia, lymphoma, plasma cell neoplasms), central nervous system (CNS) cancers (brain and spinal cord), and germ cell cancers.
Carcinoma
A carcinoma is a cancer that originates from the epithelial cells of the skin or lining of internal organs. Some types of carcinoma include squamous cell carcinoma, adenocarcinoma and hepatocellular carcinoma.3
Sarcoma
A sarcoma is a cancer of mesenchymal origin arising from the supportive or connective tissues. Soft tissue sarcoma and osteosarcoma are the two main categories of sarcoma. Some examples of sarcoma include angiosarcoma of the blood vessels, fibroblastic sarcoma and chondrosarcoma.4
Hematopoietic cancers
Hematopoietic cancers, which include leukemia and lymphoma, derive from hematopoietic cells.
Leukemia is a cancer of white blood cells (WBCs). In leukemia, the hematopoietic progenitors undergo uncontrolled expansion, resulting in an overproduction of WBCs and the inability to produce enough red blood cells and platelets. Based on the maturation of leukemic cells, leukemia is categorized into two types—acute or chronic.5
Lymphoma is a blood cancer affecting the lymphatic system and B and T cell populations of WBCs. Most lymphomas (90%) originate from B cells and the remaining 10% are T and NK cell neoplasms.6
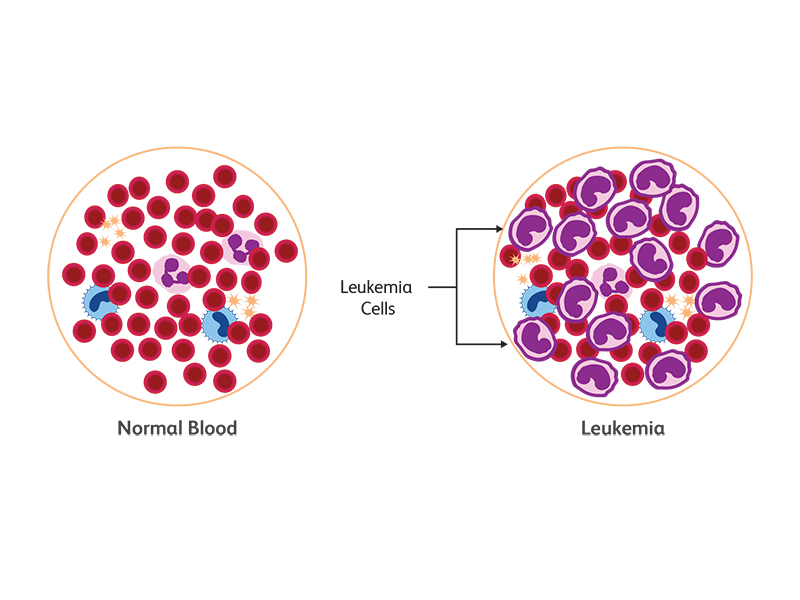
Central nervous system (CNS) cancers
CNS cancers are cancers of the brain and spinal cord that arise from the neural crest. They can be primary CNS cancers or metastatic. Some examples include primary CNS lymphoma (PCNSL), astrocytoma, oligodendroglioma, ependymoma and glioblastoma multiforme (GBM). Glioblastoma is the most common and the most aggressive primary brain tumor in adults. Even with surgical resection, chemotherapy and radiotherapy, GBM has a poor prognosis with a median survival of just over 1 year.7 Different subtypes of GBMs have been described based on the genetic alterations they harbor, such as loss of tumor suppressors (e.g., TP53, CDKN2A, PTEN) or overexpression of oncogenes (e.g., EGFRVIII).
Germ cell cancers
Germ cell neoplasia in situ (GCNIS) arise from primary germ cells (PGC) or gonocytes. PGCs develop from the epiblast and, as with gonocytes, they express pluripotent stem cell markers, e.g., KIT, NANOG. The presence of SRY will orient the differentiation into ovaries or testes. Under normal conditions, these markers progressively decrease and are replaced by markers of germ-cell commitment such as VASA and TSPY. Germ cell tumors regain the expression of stem cell- and pluripotency markers of gonocytes (e.g., OCT3/4, NANOG). GCNIS give rise to seminomas, and non-seminomas (e.g., yolk sac tumors, teratomas) differentiate from embryonic cell carcinomas.8
The tumor microenvironment (TME)
Tumorigenesis is a complex process driven by both genetic and metabolic alterations in neoplastic cells and the microenvironment surrounding these cells. TME includes blood and lymph vessels; and mesenchymal and immune cells. TME is a major contributor to tumor progression and therapy outcome. TME characteristics have been linked to response or resistance to therapy with high infiltration of cytotoxic T cells supporting a better immune response to attack tumor cells.
TME is composed of the tumor itself, associated with all its interacting cells and cellular processes—cancer stem cells, infiltrating immune cells, blood vessels that carry nutrients and signaling molecules—as well as extracellular matrix (ECM), which allows migration of cancer cells to other sites.9
The tumor immune microenvironment (TIME)
The immune signatures associated with different types of cancers have been uncovered based on genetic analyses of The Cancer Genomic Atlas (TCGA) samples, and a few common immune subtypes of cancers have been revealed. Three classes of TIME10 are described—infiltrated-excluded (IE), infiltrated-inflamed (II) and infiltrated-tertiary lymphoid structure (TLS).
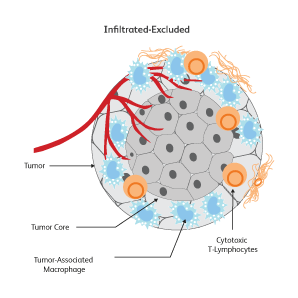
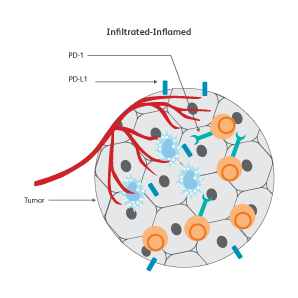
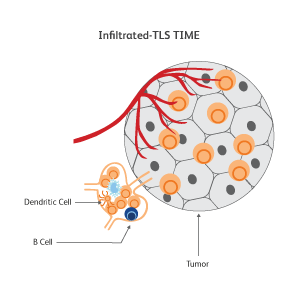
How do cancer cells shape their microenvironment?
In order to thrive, cancer cells require a well-vascularized, tolerant environment with sufficient nutrients. Cancer cells actively shape this environment to favor their growth and to protect themselves from threats from the immune system by inducing dendritic cells to exhibit tolerogenic characteristics.11
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144(5):646-674. doi: 10.1016/j.cell.2011.02.013
- Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell. 2016; 23(1):27-47. doi: 10.1016/j.cmet.2015.12.006
- Park JH, Kim JH. Pathologic differential diagnosis of metastatic carcinoma in the liver. Clin Mol Hepatol. 2019;25(1):12-20. doi: 10.3350/cmh.2018.0067
- Potter JW, Jones KB, Barrott JJ. Sarcoma-The standard-bearer in cancer discovery. Crit Rev Oncol Hematol. 2018;126:1-5. doi: 10.1016/j.critrevonc.2018.03.007
- Malouf C, Ottersbach K. Molecular processes involved in B cell acute lymphoblastic leukaemia. Cell Mol Life Sci. 2018;75(3):417-446. doi:10.1007/s00018-017-2620-z
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. doi:10.1182/blood-2016-01-643569
- Schreck KC, Grossman SA. Role of temozolomide in the treatment of cancers involving the central nervous system. Oncology (Williston Park). 2018;32(11):555-560, 569.
- Fukawa T, Kanayama HO. Current knowledge of risk factors for testicular germ cell tumors. Int J Urol. 2018;25(4):337-344. doi:10.1111/iju.13519
- Arneth B. Tumor microenvironment. Medicina (Kaunas). 2019;56(1):15. doi: 10.3390/medicina56010015
- Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541-550. doi: 10.1038/s41591-018-0014-x
- Saei A, Hadjati J. Tolerogenic dendritic cells: key regulators of peripheral tolerance in health and disease. Int Arch Allergy Immunol. 2013;161(4):293-303. doi: 10.1159/000350328>
Flow cytometry–based assays for cancer biology research
Surface and intracellular markers expressed by cancer cells can be assessed by flow cytometry to detect changes in phenotype at a single-cell level. With multicolor flow cytometry, multiple parameters can be analyzed in a single specimen or on a single cell allowing optimization of the analysis time and sample screening capabilities. Subpopulations of cancer cells, such as cancer stem cells (CSC), can also be characterized, aiding in the advancement of cancer research and therapy development.
BD Biosciences offers a comprehensive portfolio of flow cytometry instruments and reagents for cancer biology research
From specimen collection to sample preparation to cell analysis, BD Biosciences offers a multitude of tools.
Sample collection
The BD Vacutainer® products family can be used for blood cell and biomarker preservation.
Sample preparation tools
The BD Horizon™ Dri Tumor and Tissue Dissociation Reagent (TTDR) offers gentle and effective dissociation with superior epitope preservation. TTDR maximizes cell yields, while minimizing cell death, which allows effective dissociation of a variety of tumor types to enable single cell studies.
Cell staining, characterization and analysis tools
BD Biosciences offers a comprehensive portfolio of over 9,000 immunology and immuno-oncology–related research reagents that are designed for efficient characterizations of cells.
The dried reagent cocktails of BD Horizon™ Dri Panels are predesigned, ready-to-use multicolor panels optimized and tested for memory T cell, monocyte subset and TBNK cell characterization.
-
BD Horizon™ Dri Monoset Panel
Fluorochrome Marker Clone FITC CD16 4G8 PE HLA-DR L243 PerCP CD14 MΦP9 APC CD192 (CCR2) LS132.1D9 -
BD Horizon™ Dri Memory T-Cell Panel
Fluorochrome Marker Clone FITC CD197 150503 PE-Cy7 CD95 DX2 BD Horizon™ APC-R700 CD27 M-T271 APC-H7 CD3 SK7 BD Horizon™ V450 CD4 SK3 BD Horizon™ V500-C CD8 SK1 BD Horizon Brilliant Violet™ 605 CD45RA HI100 -
BD Horizon™ Dri TBNK+CD20 Reagent Panel
Fluorochrome Marker Clone BD Horizon Brilliant Violet™ 450 CD20 L27 FITC CD3 SK7 PE CD16 B73.1 PE CD56 NCAM16.2 PerCP-Cy5.5 CD45 2D1 (Hle-1) APC CD19 SJ25C1 PE-Cy7 CD4 SK3 APC-Cy7 CD8 SK1
In addition to predesigned panels, our Custom Solutions offer contract manufacturing of multicolor panels in lyophilized, liquid and/or dried formats to minimize the error(s) and time associated with manual cocktailing of reagents, increase reagent stability, and significantly enhance performance consistency.
The BD Horizon Brilliant™ Polymer Dyes were developed from advanced Sirigen dye technology, enabling high-parameter flow cytometry experiments for discerning cell populations. The bright dyes help in distinguishing dim cell populations, such as tumor-infiltrating lymphocytes or cells that have few receptors on the surface from other cells in a sample.
From sorting to analysis, our cell sorters, cell analyzers and downstream sequencing and informatics analysis tools provide efficient solutions for cancer biology research.
Our research cell analyzers, such as BD FACSymphony™ Flow Cytometer, can be used for the simultaneous measurement of up to 50 parameters. The BD LSRFortessa™ System can analyze up to 20 parameters.
Our multiomic solutions include the BD Rhapsody™ Single-Cell Analysis System, which enables high-throughput capture and analysis of hundreds of single cells. Our single-cell multiomics reagents portfolio enables the analysis of the entire transcriptome or of targeted genes of interest. Targeted panels like the BD Rhapsody™ Immune Response Panel allow profiling of specific immune cell types.
Sample data generated using the BD Biosciences cancer biology tools
Expression of immune checkpoint receptors on peripheral blood immune cells using a 10-color assay on the BD FACSLyric™ Flow Cytometer.
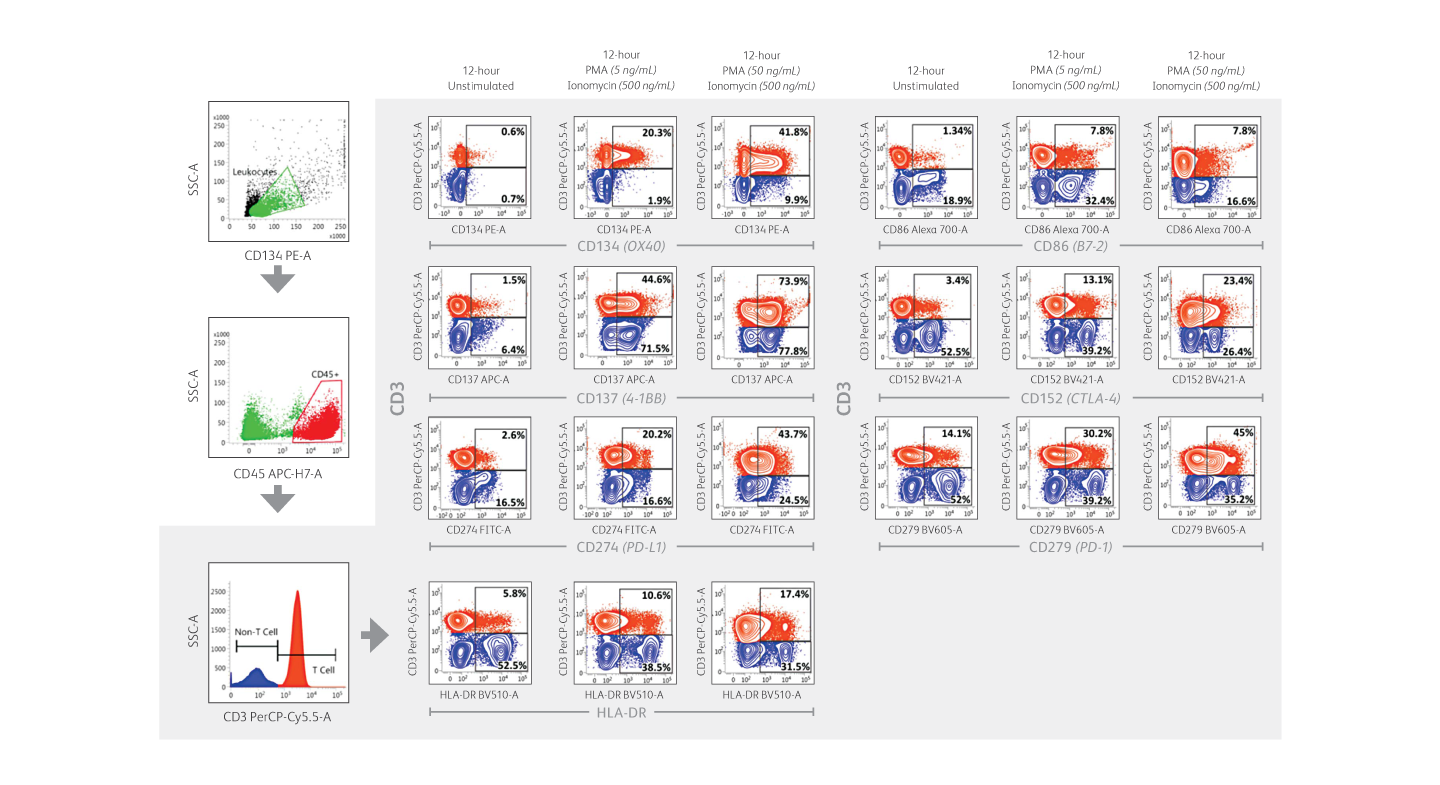
Demonstration of immune checkpoint receptor expression on activated T cells that are regulated in part by time-in-culture or by stimulatory conditions using the BD FACSLyric™ Analyzer. Intermediate concentrations (50 ng/mL) of phorbol 12-myristate 13-acetate (PMA) plus ionomycin appeared to induce robust upregulation of CD134, CD137, PD-L1/CD274, HLA-DR, CD86, CD152 and PD-1/CD279 in CD3+ T cells.
Analysis of PD-1 expression on activated T cells using PE-EH12.1 as the detection reagent.
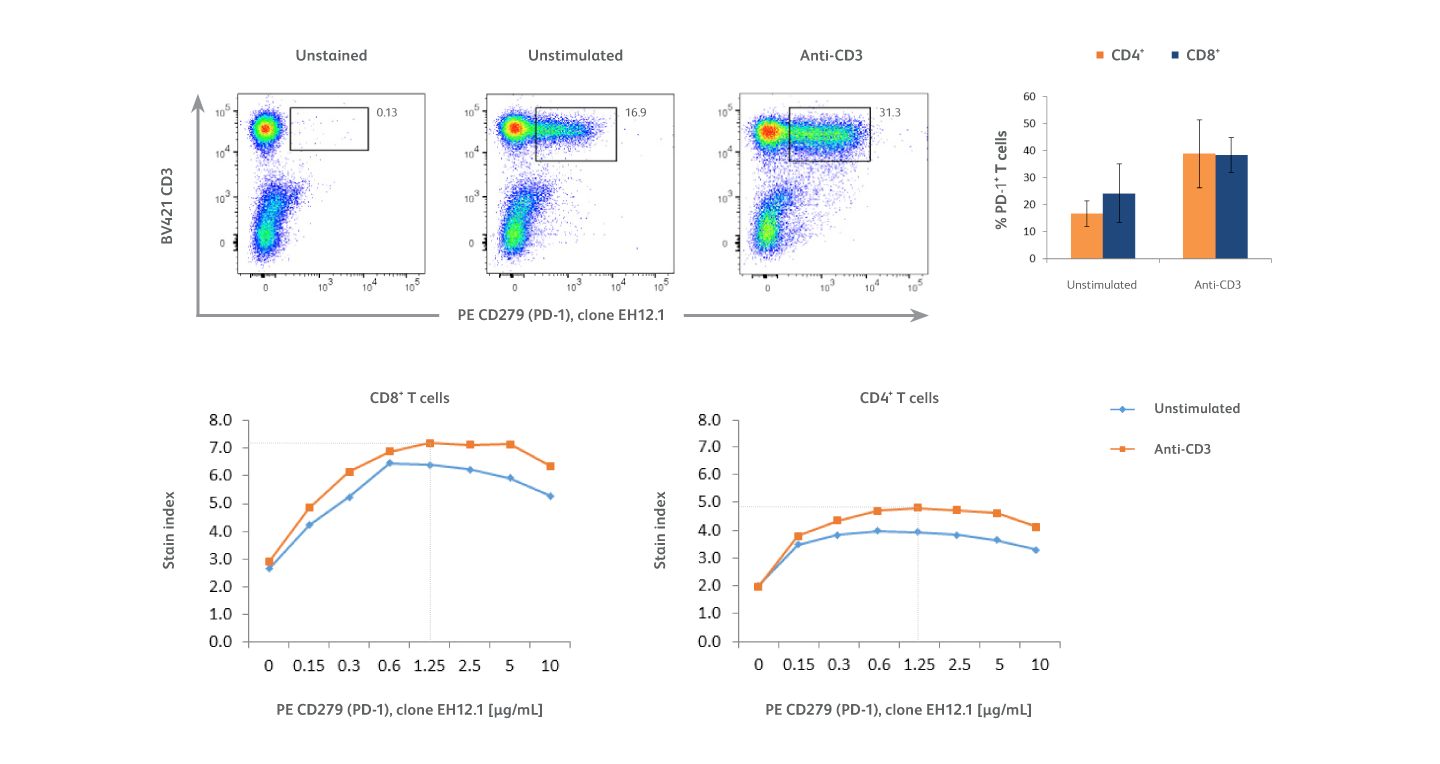
PMBCs were stimulated overnight with 10 μg/mL of immobilized BD Pharmingen™ Purified NA/LE Mouse Anti-Human CD3 Antibody or cultured in media alone (unstimulated cells). Unstimulated and anti-CD3 stimulated cells were pre-incubated with BD Pharmingen™ Human BD Fc Block™ Reagent and stained with BD Horizon Brilliant Violet™ 421 (BV421) Anti-Human CD3 Antibody, BD Horizon Brilliant™ UV 395 (BUV395) Anti-Human CD4 Antibody and serial two-fold dilutions (ranging from 0.15 μg/mL to 10 μg/mL) of BD Pharmingen™ PE Mouse Anti-Human CD279 (PD-1) Antibody, clone EH12.1 (PE-EH12.1). Top left: Representative data from one donor showing expression of PD-1 and CD3 on lymphocytes (gated using forward and side scatter, not shown). Top right: Bar graph represents percentages of PD-1⁺ T cells among CD4⁺ (orange) and CD8⁺ (blue) T-cell subsets in samples from six donors that were stimulated with anti-CD3 or left unstimulated. Bottom panel: Titration curves show the optimal concentration of PE-EH12.1 (1.25 μg/mL) yielding the highest stain index on CD4⁺ and CD8⁺ T cell subsets in the different culture conditions. Overall, results show that anti-CD3 stimulation increased the percentages of T cells expressing PD-1 among both CD4⁺ T and CD8⁺ T cell subsets, with PD-1 expression higher on CD8⁺ T cells.
Unless otherwise noted, BD products described in this page are For Research Use Only. Not for use in diagnostic or therapeutic procedures.
BD flow cytometers are Class 1 Laser Products.
The BD FACSLyric™ Flow Cytometer is for Research Use Only with BD FACSuite™ Application for up to 12 colors. Not for use in diagnostic or therapeutic procedures.