-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD Rhapsody™ TCR/BCR Profiling Assays for Human and Mouse
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Immunohistochemistry
Immunohistochemistry (IHC) is a powerful tool that uses the ability of specific antibodies to detect antigens of interest in tissue samples. IHC is widely used in the pathological evaluation of cancer tissues, to predict the prognosis of tumors, to predict responses to therapy, to confirm presence of infections, and in numerous research applications.1 In addition to detecting the presence or absence of an antigen, IHC also allows the determination of antigen distribution and location.
Principles of immunohistochemistry
The tissue of interest is first fixed, paraffin embedded or cryopreserved, and sectioned. The stored sections are processed (using deparaffinization for paraffin-embedded sections or by heating for cryopreserved sections) for antigen retrieval and then immunostained using antibodies specific to the antigens. Just like western blotting or ELISA, IHC can be direct or indirect (using only one antibody or a combination of a primary and a secondary antibody). Detection is usually accomplished by using enzymes such as horseradish peroxidase (HRP) or alkaline phosphatase (AP). Samples are visualized using microscopy.
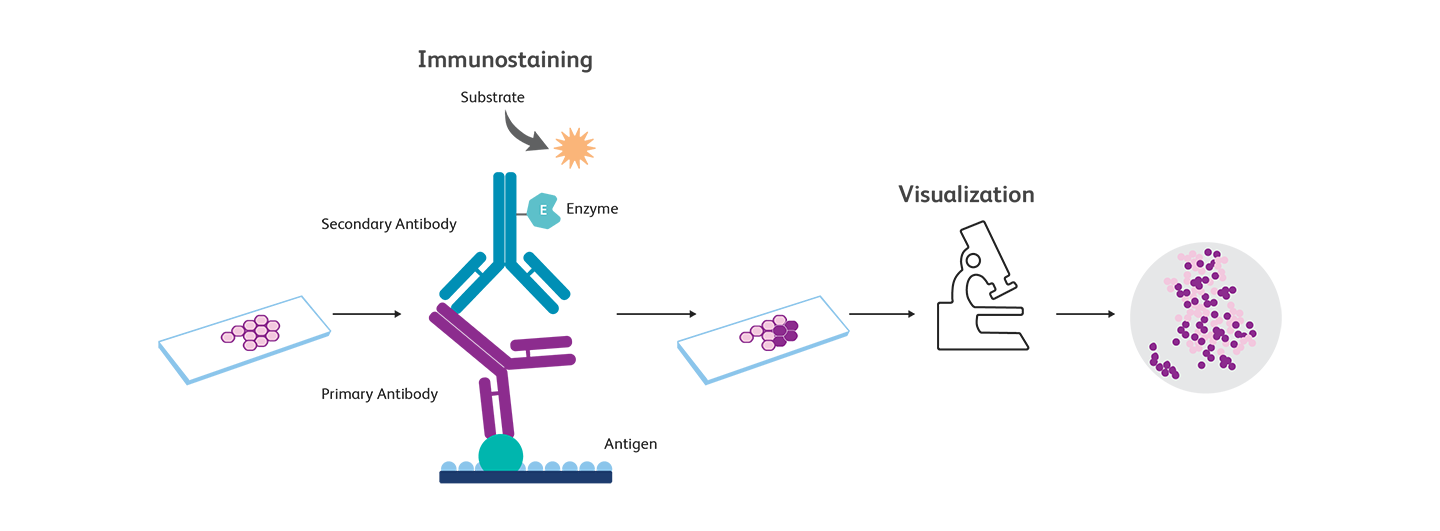
Immunocytochemistry, immunohistochemistry and immunofluorescence—what are the differences?
The principles of immunohistochemistry are similar to those of immunocytochemistry (ICC), but IHC uses tissue samples while ICC uses cells.
Both immunocytochemistry (ICC) and immunohistochemistry (IHC) employ immune labeling of antigens for analysis and use immunofluorescence methods. Sometimes, immunocytochemistry and immunofluorescence are used interchangeably.
While ICC focuses on analysis at a cellular level, IHC allows examination of the whole tissue. Both techniques use immunofluorescence for detection. It is also possible to use enzyme-based detection (for example, using 3,3’-diaminobenzidine (DAB)).
References
- Duraiyan J, Govindarajan R, Kaliyappan K, Palanisamy M. Applications of immunohistochemistry. J Pharm Bioallied Sci. 2012;4(Suppl 2):S307-S309. doi: 10.4103/0975-7406.100281
Sample preparation for IHC
Once the tissue sample is obtained it is critical to preserve it in order to keep the tissue architecture as intact as possible and to keep the antigen as close as possible to the state it was at the time of collection. The choice of preparation will also affect the storage of samples over time and the ease of detection of antigens.
The choice of fixative depends on the antigen to be detected as well as the type of analysis (e.g., morphology or expression).
Tissue fixation
Using 4% paraformaldehyde (PFA) is a method routinely used in immunohistochemistry to preserve tissue samples. While this fixation method allows good preservation of tissue architecture, it can cause conformational changes that have the potential to affect proper detection of antigen epitopes. Because of formaldehyde-induced epitope masking, antigen retrieval agents are usually used to increase the antigen access potential.
As an alternative to formaldehyde, a mild fixative, such as zinc can be used for paraffin-embedded sections. In addition to formaldehyde and zinc, alcohol or acetone can also be used for fixing IHC samples.
Tissue embedding
After tissue samples have been fixed, the tissue samples are paraffin embedded to enable tissue sectioning. Paraffin is generally used to prepare blocks for sectioning. Another tissue preparation method is snap freezing of the sample. While freezing is a quick method and allows preservation of antigen epitopes, ice crystals formed during freezing can alter tissue architecture.
Tissue sectioning
Tissue sectioning allows the generation of slices that will be used as a staining ground for the immunohistochemistry experiment. Paraffin-embedded tissues will require a microtome for sectioning whereas frozen tissues will be sectioned using a cryostat.
BD Pharmingen™ IHC Zinc Fixative is a mild fixative that leaves antigen and tissue morphology intact, which makes it particularly appropriate for leukocyte marker detection and yields the same architecture preservation of formaldehyde fixed tissues. Combining BD Pharmingen™ IHC Zinc Fixative with BD Pharmingen™ Retrievagen A (pH 6.0) and Retrievagen B (pH 9.5) Solutions will further enhance antibody reactivity for some epitopes.
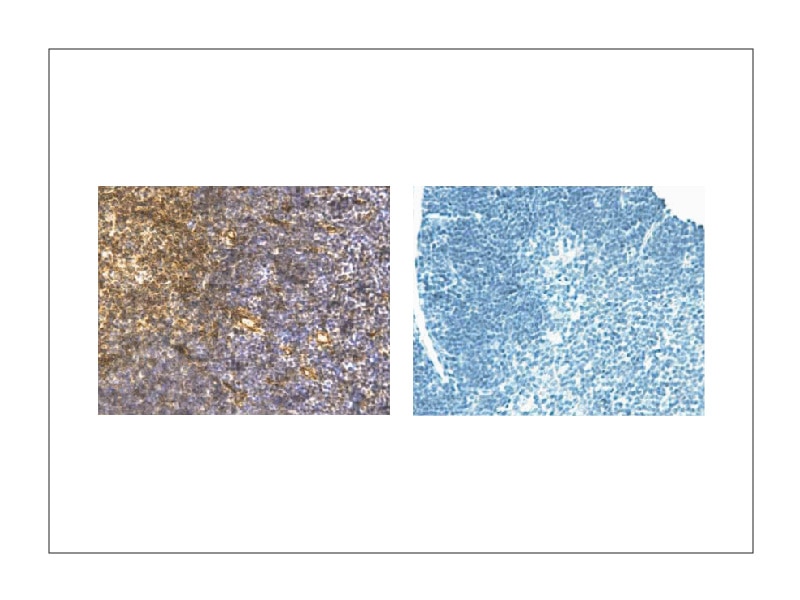
Antigen retrieval methods for IHC
Two main antigen retrieval methods are used to reveal antigen epitopes masked because of embedding and to increase epitope immunoreactivity:1
Protease-induced epitope retrieval (PIER)
Protease-induced epitope retrieval (PIER), also known as enzymatic retrieval, uses protease chemical reaction to undo cross-links that have been created during tissue fixation. This method is usually employed to retrieve epitopes that are difficult to restore. It uses buffer solutions of enzymes (e.g., trypsin, proteinase K) that are around neutral pH. As it is a harsh retrieval method with non-specific enzymatic reactions, over-incubation can damage tissue architecture along with the epitope of interest.
Heat-induced epitope retrieval (HIER)
Heat-induced epitope retrieval (HIER) relies on heat and pressurization to retrieve antigenic epitopes. While PIER uses neutral buffer solutions, in HIER a range of pH can be used depending on the target antigen.
BD Biosciences offers two effective antigen retrieval reagents—BD Pharmingen™ Retrievagen A (pH 6.0) and Retrievagen B (pH 9.5) Solutions—that are particularly suited for use in combination with tissue fixed with BD Pharmingen™ IHC Zinc Fixative.
References
- Ireka Y, Agustina H, Aziz A, Hernowo BS, Suryanti S. Comparison of fixation methods for preservation cytology specimens of cell block preparation using 10% neutral buffer formalin and 96% alcohol fixation in E-cadherin and Ki-67 immunohistochemical examination. Open Access Maced J Med Sci. 2019;7(19):3139-3144. doi: 10.3889/oamjms.2019.452.
Direct and indirect immunohistochemistry methods
Two main immunostaining methods have been described:
Direct method
Direct immunohistochemistry uses a single-conjugated antibody to detect the antigen epitope of interest. This method is used for the detection of very abundant antigens that do not need any amplification.
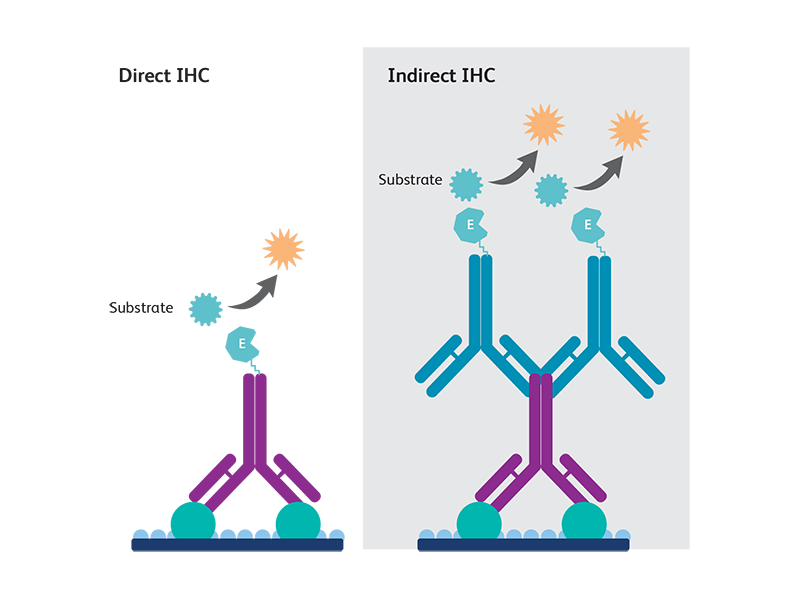
Indirect method
Indirect immunohistochemistry uses two steps of antibody staining—a primary antibody against the antigen of interest is first bound to the antigen, then a secondary antibody conjugated against the primary antibody species is used to generate signals detectable by microscopy. Indirect immunohistochemistry is widely used as it allows a better signal detection for mid- to lowly- expressed antigens.
The choice of method used depends on several factors, such as antigen abundance, distribution and how the tissue samples were prepared.1
IHC detection methods
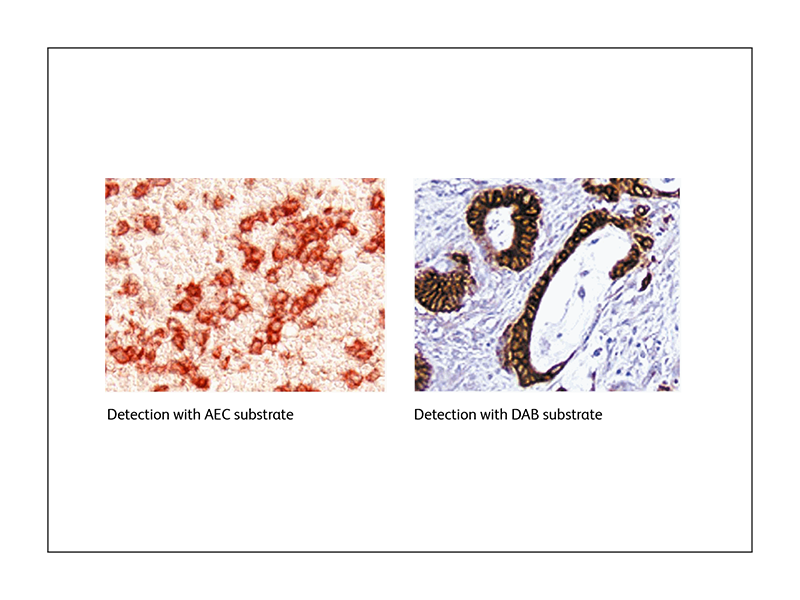
Colorimetric detection
Colorimetric or chromogenic immunohistochemistry uses the reaction between an enzyme and its substrate to generate a chromogenic precipitate that localizes at the site of reaction. The two main enzymatic detection systems used are horseradish peroxidase (HRP) and alkaline phosphatase (AP). BD Biosciences offers a range of substrates that can be used with these enzymes, such as the BD Pharmingen™ DAB Substrate Kit and the BD Pharmingen™ AEC Substrate Kit.
Fluorescence detection
Fluorescence immunohistochemistry uses antibodies coupled to a fluorophore that, when excited by the proper wavelength of light, emit a fluorescence signal that can be visualized by a fluorescence microscope. BD Biosciences offers a range of fluorescence-coupled secondary antibodies for IHC.
Improving immunohistochemistry staining quality
To obtain better visualization of your immunohistochemistry staining, you should plan your experiment carefully, start with good tissue collection, and prepare your sample choosing the options that are suited to optimally detect your target antigen.
Signal amplification
Signals can be amplified using polyclonal antibodies that can recognize multiple epitopes of a single antigen. Secondary antibody conjugated with multiple complexes, as well as other methods such as polymer-based method, avidin-biotin complex (ABC) method and labeled-streptavidin biotin (LSAB) method, can also be used.
Reduction of nonspecific binding
BD Biosciences provides blocking solutions to help reduce nonspecific binding and increase signal-to-noise ratio. Reagents to keep background noise at check during staining such as the BD Pharmingen™ Antibody Diluent for IHC are also available.
Sample IHC data generated using BD Biosciences antibodies
In addition to antibodies and reagents, BD Biosciences also offers validated protocols for IHC.
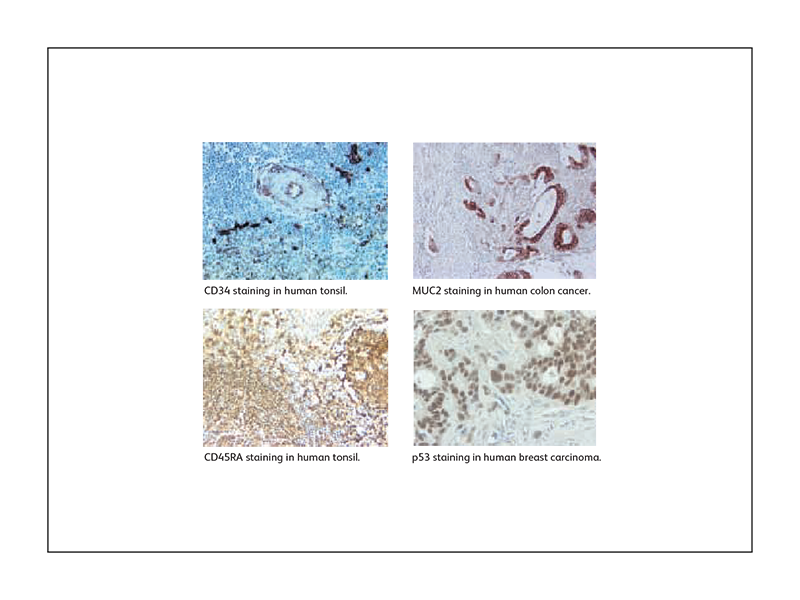
References
- Magaki S, Hojat SA, Wei B, So A, Yong WH. An introduction to the performance of immunohistochemistry. Methods Mol Biol. 2019;1897:289-298. doi: 10.1007/978-1-4939-8935-5_25