-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

BD OneFlow™
Acute Leukaemia Orientation Tube (ALOT)
Intended as an aid in the diagnosis of acute lymphoblastic leukaemia and non-lymphoid acute leukaemia
For Professionals in Clinical Diagnostics
Overview
The BD OneFlow™ ALOT is part of the innovative BD OneFlow™ Solution, a comprehensive set of reagents, setup beads, protocols and assay templates to reproducibly set up the flow cytometer and stain, acquire and analyse patient specimens for immunophenotyping of normal and aberrant cell populations. It is built on the research and validation work of the EuroFlow™ Consortium on the characterisation of hematological malignancies for improved accurate diagnosis.1*
The BD OneFlow™ ALOT is intended for flow cytometric immunophenotyping of aberrant immature populations of hematopoietic cells (lymphoid and non-lymphoid lineage) in bone marrow and peripheral blood as an aid in the diagnosis of acute lymphoblastic leukaemia and non-lymphoid acute leukaemia.
Other reagents belonging to the BD OneFlow™ Solution are BD OneFlow™ Lymphoid Screening Tube (LST), BD OneFlow™ B-cell Chronic Lymphoproliferative Diseases Tube 1 (B-CLPD T1), BD OneFlow™ Plasma Cell Screening Tube (PCST), BD OneFlow™ Plasma Cell Disorders (PCD) Tube.
FEATURES
The BD OneFlow™ ALOT:
Is a pre-configured single-use, 8-color reagent
Comprises 2 ready-to-use tubes, one containing the cytoplasmic markers (C tube) and the other containing the surface markers (S tube)
Can guide the need for further analysis as a screening tube in combination with panel(s) specifically designed for the classification of different forms of B cell, T cell and myeloid acute leukaemias
Is available in 10 test/box size (4 pouches of 5 tubes each: 2 pouches of S tubes and 2 pouches of C tubes)
Allows for easy visual identification as dark red color-coded boxes, pouches and tubes
The BD OneFlow™ Reagents increase efficiency by simplifying instrument standardisation, reducing the time to prepare the instrument as well as the technical burden and training needs.2
For BD FACSLyric™ Flow Cytometer users:
BD® CS&T IVD Beads standardise setup and monitoring for consistent performance and ensure reproducibility through Universal Setup
BD® FC Beads (7-Color, 5-Color and 2-Color Kits) support consistency of results, eliminating the need for using cells for compensation
BD® CS&T Beads and BD® FC Beads kits necessary for instrument setup and compensation can be used for other CE-IVD assays on the BD FACSLyric™ Flow Cytometer
For BD FACSCanto™ II Flow Cytometer users:
BD FACSDiva™ CS&T IVD Beads standardise setup and monitoring for consistent performance and ensure reproducibility with Application Settings
BD OneFlow™ Setup Beads ensure data accuracy and reproducibility by providing assay-specific target values, as per EuroFlow™ SOPs3
Specifically designed BD® FC Beads eliminate the need for using single-vial reagents and cells for compensation
The BD OneFlow™ ALOT reagent composition
| Antibody | Fluorochrome | Clone | Tube | Target Populations |
|---|---|---|---|---|
| MP0 | FITC | MP0-7 | C | Myeloid lineage marker |
CD79A | PE | HM57 | C | B-lineage marker |
| CD34 | PerCP-Cy5.5 | 8G12 | S | Backbone marker (BCP-ALL and AML Panels). Identification of immature cells. |
| CD19 | PE-Cy7 | SJ25-C1 | S | Backbone marker (BCP-ALL Panel). B-lineage marker |
| CD7 | APC | M-T701 | S | T-lineage marker |
| CD3 | APC-H7 | SK7 | S | Backbone marker (T-ALL panel). |
| CD3 | BD Horizon™ V450 | UCHT-1 | C | Backbone marker (T-ALL panel). Maturity marker for T-cells. |
| CD45 | BD Horizon™ V500-C | 2D1 | S | Backbone marker. Identification of immature cells. |
The EuroFlow antibody panel article1* has a full description of the utility of the antibodies chosen for the BD OneFlow™ ALOT.
APPLICATIONS
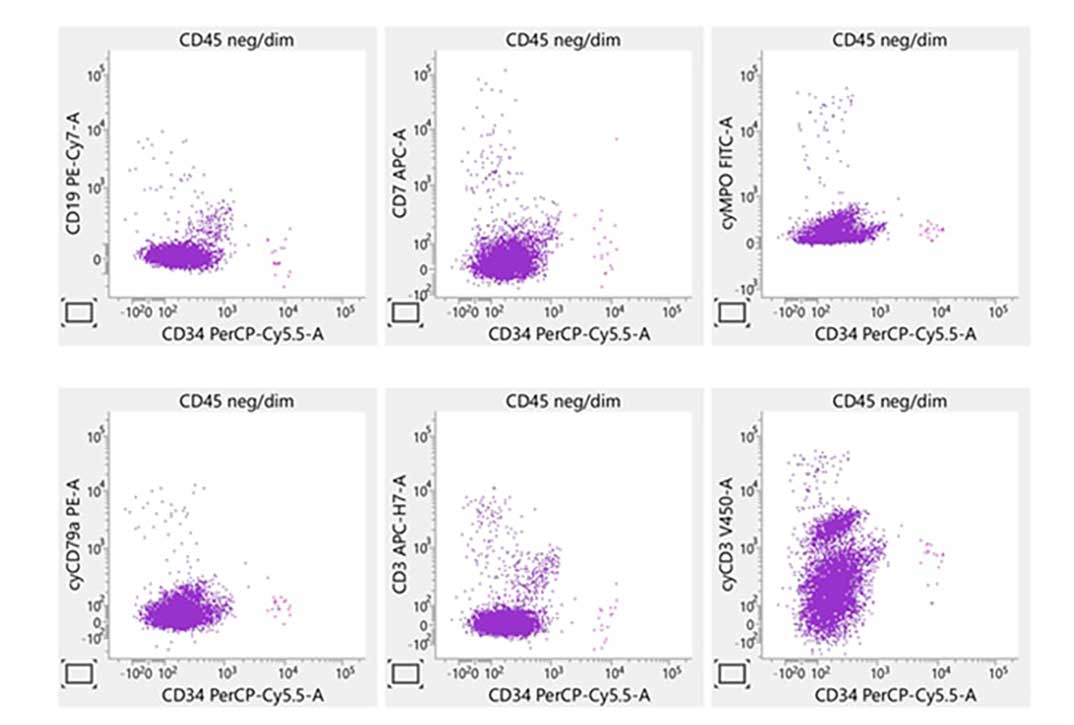
Laboratory report of a normal peripheral blood specimen stained with the 8-color 8-antibody BD OneFlow™ ALOT single-test reagent acquired on the BD FACSLyric™ Flow Cytometer with BD FACSuite™ Clinical Application v1.4 for the identification of aberrant immature lymphoid and non-lymphoid populations.
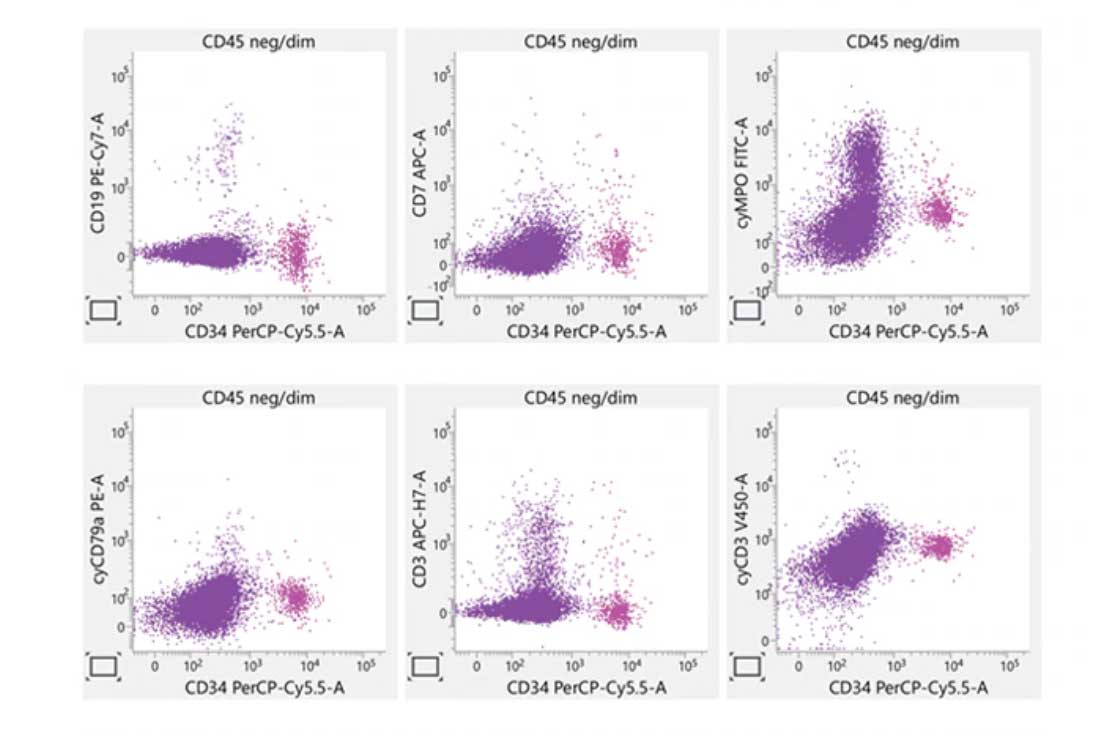
Laboratory report of an abnormal bone marrow specimen stained with the 8-color 8-antibody BD OneFlow™ ALOT single-test reagent acquired on the BD FACSLyric™ Flow Cytometer with BD FACSuite™ Clinical Application v1.4 for the identification of aberrant immature lymphoid and non-lymphoid populations.

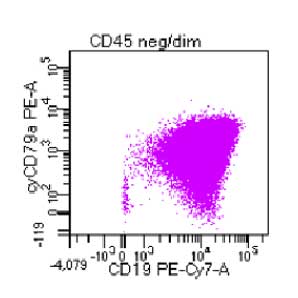
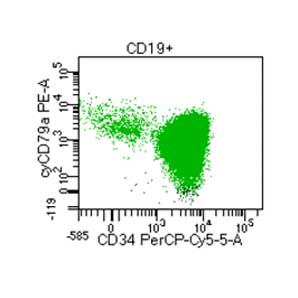
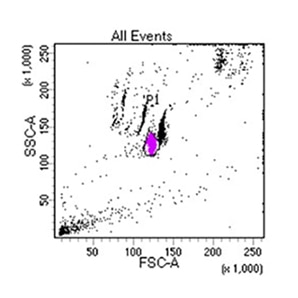
Supporting Evidence
“An unprecedented orientation efficiency of 98.3% for non-ambiguous lineage cases was shown for the [EuroFlow] ALOT combination with a series of 483 newly diagnosed acute leukemia cases, tested prospectively at different centers.”1*
Results from the clinical trial showed that the BD OneFlow™ ALOT gave an overall agreement of 100% (93 of 93) in orienting patients into lymphoid (44 of 44 concordant) and non-lymphoid (49 of 49 concordant) lineages when compared with the EuroFlow system. Furthermore, interpretation of clinical trial results using the BD OneFlow™ Solution was highly concordant to diagnostic truth derived from a composite of patient history and lab findings.
-
Instructions for Use
-
Application Guides - For BD FACSLyric™ Flow Cytometer Users
-
Applications Guides - For BD FACSCanto™ II Flow Cytometer Users
-
Tools
-
Application Note
-
Application Guides - For BD FACSLyric™ Flow Cytometer Users
-
Application Guides - For BD FACSCanto™ II Flow Cytometer Users
References
- van Dongen JJM, Lhermitte L, Böttcher S, et al. on behalf of the EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9): 1908-1975. doi: 10.1038/leu.2012.120
- Moloney E, Watson H, Barge D, et al. Efficiency and health economic evaluations of BD OneFlow™ Flow Cytometry Reagents for diagnosing chronic lymphoid leukemia. Cytometry B Clin Cytom. 2019;96(6):514-520. doi: 10.1002/cyto.b.21779
- Kalina T, Flores-Montero J, van der Velden VHJ, et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 2012;26(9):1986-2010. doi: 10.1038/leu.2012.122
The BD FACSLyric™ and BD FACSCanto™ II Flow Cytometers are Class 1 Laser Products.
BD OneFlow™ Reagents, BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ Applications, BD FACSCanto™ II Flow Cytometer, BD® FC Beads (8-Color, 7-Color, 5-Color and 2-Color Kits), BD OneFlow™ Setup Beads, BD FACSDiva™ CS&T IVD Beads and BD® CS&T Beads are CE marked in compliance with the European In Vitro Diagnostic Medical Device Directive 98/79/EC.
The BD OneFlow™ Solution is intended for professional use only.
The EuroFlow trademark is the property of the EuroFlow Consortium and cannot be reproduced or published without prior written permission from the EuroFlow coordinator (www.euroflow.org).
Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
*This publication refers to EuroFlow and is relevant to the BD OneFlow™ Solution as the BD OneFlow™ Solution demonstrated equivalent results to EuroFlow through a clinical trial.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.