-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Fluorochrome Faceoff
Watch your favourite fluorochromes take on the competition!
Game 5: Buffer Compatibility
-
Once you determine the markers you want to investigate in your flow cytometry panel and the protocol you want to use, you will need to determine what fluorochromes are compatible with the staining, fixation and permeabilisation buffers you will use.
If you are interested in investigating markers within the cell, your cells may need to undergo treatments to make those antigens accessible to your staining reagents. It is important to consider how using different buffers affects the staining pattern of your markers and biological resolution of your data.
Here we demonstrate an example using a common fixation and permeabilisation protocol. The staining of several fluorochromes excited by the yellow-green laser with peak emission around 610 nm is evaluated in cells treated with fixative and methanol-based permeabilisation buffer after surface staining (blue line). Untreated cells are used as a reference (red line).
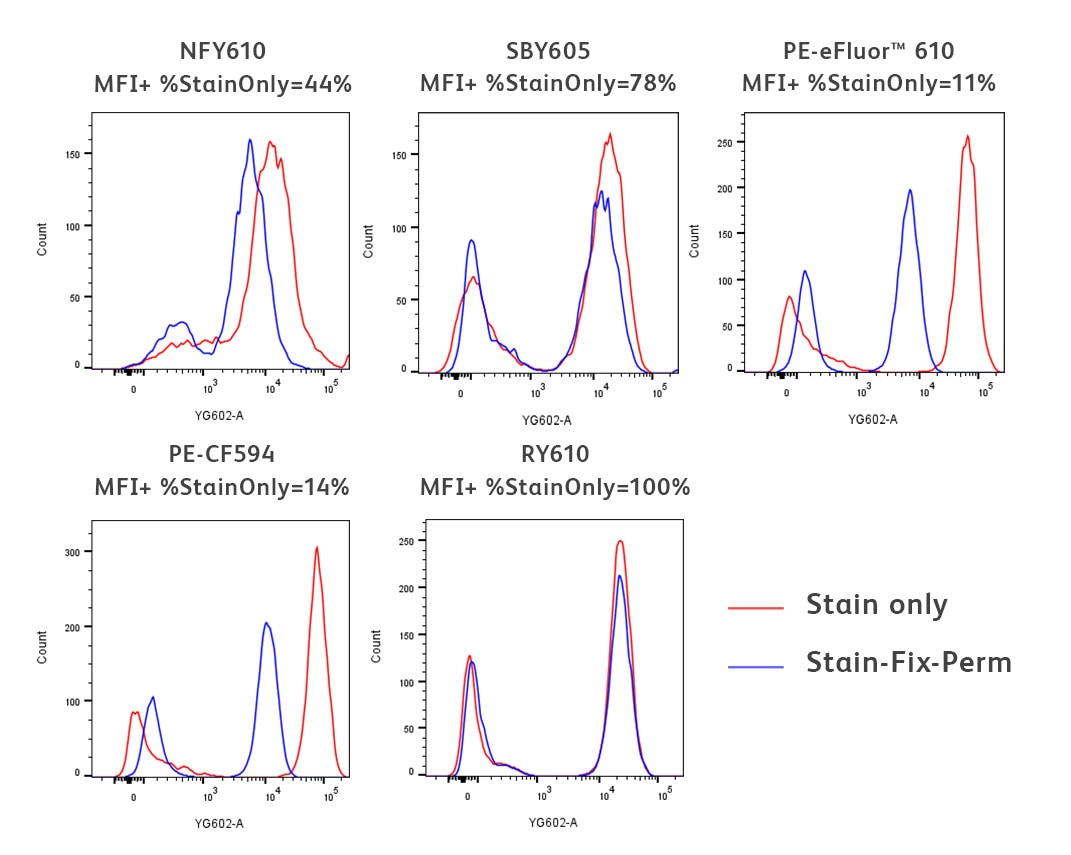
Freshly isolated human PBMCs were stained for 30 minutes with either BD Horizon™ RY610 Mouse Anti-Human CD3 UCHT1 (Cat. No. 571134), BD Horizon™ PE-CF594 Mouse Anti-Human CD3 UCHT1 (Cat. No. 562280), Thermo Fisher NovaFluor™ Yellow 610 Mouse Anti-Human CD3 UCHT1, Bio-Rad StarBright™ Yellow 605 Mouse Anti-Human CD3 UCHT1 or Thermo Fisher PE-eFluor™ 610 Mouse Anti-Human CD3 UCHT1 Reagent. The stained PBMCs were then washed twice with BD Pharmingen™ Stain Buffer (Cat. No. 554656) (red histograms). For the treated condition (blue histograms), after washing twice with BD Pharmingen™ Stain Buffer, cells were fixed using BD Cytofix™ Fixation Buffer (Cat. No. 554655) and permeabilised with BD Phosflow™ Perm Buffer III (Cat. No. 558050) for 30 minutes on ice. Cells were then washed twice with BD Pharmingen™ Stain Buffer. Histograms were derived from gated events based on light scattering characteristics for lymphocytes. Flow cytometry and data analysis were performed using a BD FACSymphony™ A5 SE Cell Analyser System and FlowJo™ Software.
Winner’s Circle
You can tell if the biological resolution of your data is impacted by the buffer you’re using by comparing the staining profiles of the untreated (red) and fix-perm treated samples (blue). As you can see in the data above, there is varying impact to the fluorochromes used in this experiment, from little-to-no impact for RY610 and StarBright™ Yellow 605 to a significant reduction in signal for PE-CF594 and PE-eFluor™ 610.
PRO TIP: It is important to note that PE-tandem dyes are notorious for being impacted by these harsher staining conditions.
Runner-Up:
NFY610
Request a sample of RY610
*Required fields
Disclaimer: Results and conclusions shown throughout the Fluorochrome Faceoff are based on experiments performed under the conditions described. Users should evaluate reagents with their specific protocols as results may vary with different experimental conditions.
The Importance of Buffer Compatibility
Once you determine the markers you want to investigate in your flow cytometry panel and the protocol you want to use, you will need to determine what fluorochromes are compatible with the staining, fixation and permeabilisation buffers you will use.
If you are interested in investigating markers within the cell, your cells may need to undergo treatments to make those antigens accessible to your staining reagents. Whether studying markers in the cytoplasm or nucleus, one will need to use various buffers for fixation and permeabilisation. It is important to consider how using different buffers affects the staining pattern of your markers and biological resolution of your data.
BD flow cytometers are Class 1 Laser Products. For Research Use Only. Not for use in diagnostic or therapeutic procedures.
CF is a trademark of Biotium, Inc. Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.