-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?



Set Your Flow Cytometry Up for Success
Dive into our comprehensive resources to master the art of panel design. Whether you’re a seasoned researcher or a curious explorer, we’ve got you covered

Cytognos Products Now Available Online!
Explore the integrated BD-Cytognos portfolio, enhance your diagnostic and research capabilities and the journey from diagnosis to monitoring and disease follow-up

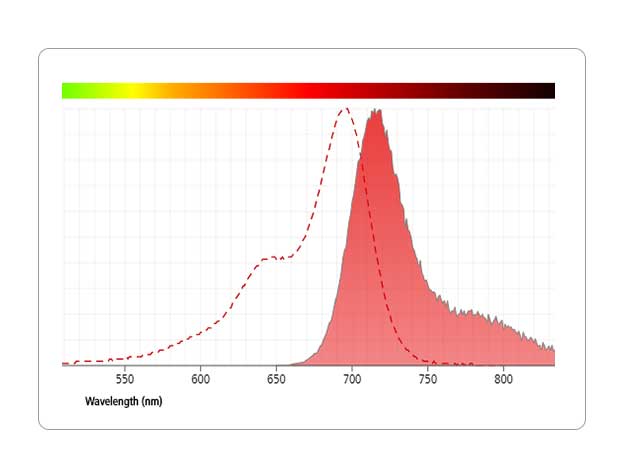
Catch the Full Spectrum
Use spectral flow cytometry to advance your high parameter research


Applications and Solutions
A comprehensive suite of trusted products, integrated solutions, useful tools and a wealth of information to advance your flow cytometry applications.

Request a Free Sample
of our New Blue-laser Fluorochromes
Take a step closer to maximizing panel flexibility for spectral flow cytometry. Try out BD Horizon RealBlue™ 705 (RB705)
or BD Horizon RealBlue™ 744 (RB744).
Innovative solutions to power your diagnostics and research
Backed by cutting-edge technology and more than 45 years of flow cytometry expertise
“My order was placed quickly and easily. No technology problems. The new BD Biosciences website worked flawlessly! It saved me a lot of time. The new experience is like taking a walk in the park. It’s like night and day!”

“My order was placed quickly and easily. No technology problems. The new BD Biosciences website worked flawlessly! It saved me a lot of time. The new experience is like taking a walk in the park. It’s like night and day!”
“I’m really excited about the BD Horizon RealBlue™ 780 Dye. The staining we have done so far with Ki-67 and Granzyme B can be seen very clearly, even with high dilutions.”

“I’m really excited about the BD Horizon RealBlue™ 780 Dye. The staining we have done so far with Ki-67 and Granzyme B can be seen very clearly, even with high dilutions.”
“BD is positioned with BD® CS&T and BD® FC Bead technology to enable instrument standardization simply from day to day, instrument to instrument, and lab to lab. Reference control–based instrument standardization is the next step for flow cytometry.”

“BD is positioned with BD® CS&T and BD® FC Bead technology to enable instrument standardization simply from day to day, instrument to instrument, and lab to lab. Reference control–based instrument standardization is the next step for flow cytometry.”
“At last, a fully integrated end-to-end walkaway solution, which is going to make our lives significantly easier. It reduces errors in both the pre-analytical and analytical phases, leading to increased productivity and efficiency, but most importantly provides a comprehensive fully audit-able account of the whole process. Finally, a solution that creates capacity to allow us to meet our key performance indicators.”

“At last, a fully integrated end-to-end walkaway solution, which is going to make our lives significantly easier. It reduces errors in both the pre-analytical and analytical phases, leading to increased productivity and efficiency, but most importantly provides a comprehensive fully audit-able account of the whole process. Finally, a solution that creates capacity to allow us to meet our key performance indicators.”
"BD Horizon RealYellow™ 586 (RY586) Reagents look like the best alternative for PE and will simplify panel design on conventional flow cytometers. They can also be an additional dye for spectral panel design.”

"BD Horizon RealYellow™ 586 (RY586) Reagents look like the best alternative for PE and will simplify panel design on conventional flow cytometers. They can also be an additional dye for spectral panel design.”


Utilize our featured resources with invaluable information to advance your science.
Explore a variety of tools for your multicolor flow cytometry, from panel design to selection tools—an array of aids to support your research.
Be part of the flow cytometry community with the latest flow cytometry news, thought leader opinions, blogs on breakthrough research, interesting flow cytometry publication reviews, and more.
Use our excellent product and technical support with our >45 years of flow cytometry expertise and support resources.
Find a seamless customer experience and an easy way to create and manage accounts.
Explore a variety of ways that work with your purchasing process to place and track orders.
Explore our webinar series spanning a broad range of topics and in-depth reviews by senior scientists, professionals and clinicians.
Use a variety of training courses for instruments and applications to take full advantage of the capabilities of BD products.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.