-
Your selected location is
Middle East / Africa
- Change location/language
-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD OMICS-One™ WTA Next Assay
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Forward, Together
The acquisition of Cytognos accelerates the expansion of the BD portfolio in blood cancer diagnostics, immune assessment tests and informatics, by addressing the needs of clinicians and care providers to better understand the immune system, immune response and minimal residual disease (MRD).
Cytognos' key products increase BD diagnostic and research solutions for certain types of cancers that use flow cytometry as the primary means of discovery, diagnosis and understanding, including lymphoma, leukaemia and multiple myeloma.
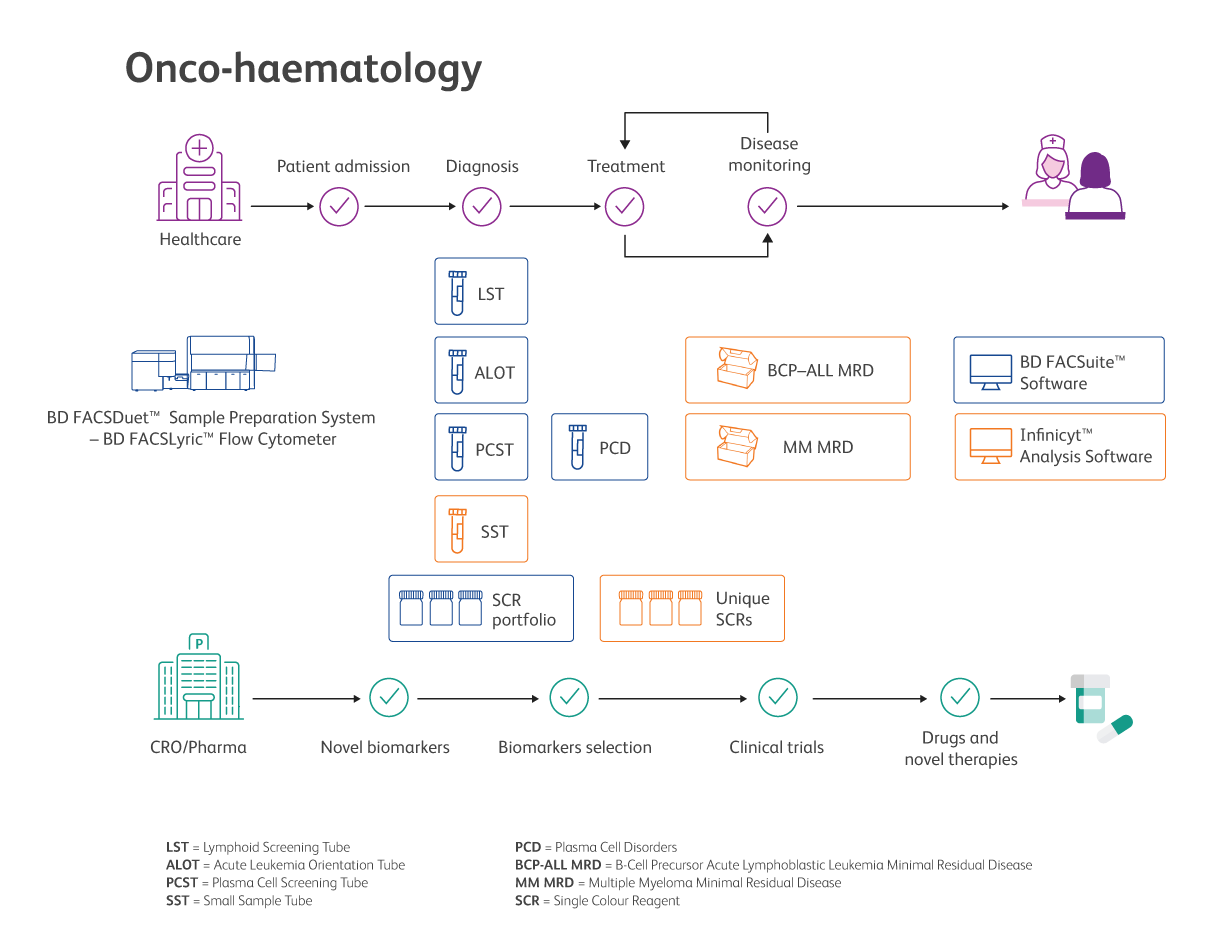
The synergy of Cytognos and BD for Onco-haematology
Onco-haematology at Cytognos
At Cytognos, we are committed to bringing solutions to improve patient care and laboratory efficiency by next generation flow cytometry.
Cytognos offers scientific-based solutions to monitoring patients affected by some forms of leukemia, alerting clinicians if the disease is still present or if there is a sign of recurrence. This is a key element of the patient care continuum, helping clinicians in the timely treatment of patients.
Onco-haematology at BD
Built on the research and validation work of the EuroFlow™ Consortium on the characterisation of hematological malignancies for improved accurate diagnosis1, BD brings the standardisation of leukaemia and lymphoma immunophenotyping one step forward, improving laboratory efficiency and enabling reliability and accuracy of results for clinical decisions and ultimately patient outcomes2,3.
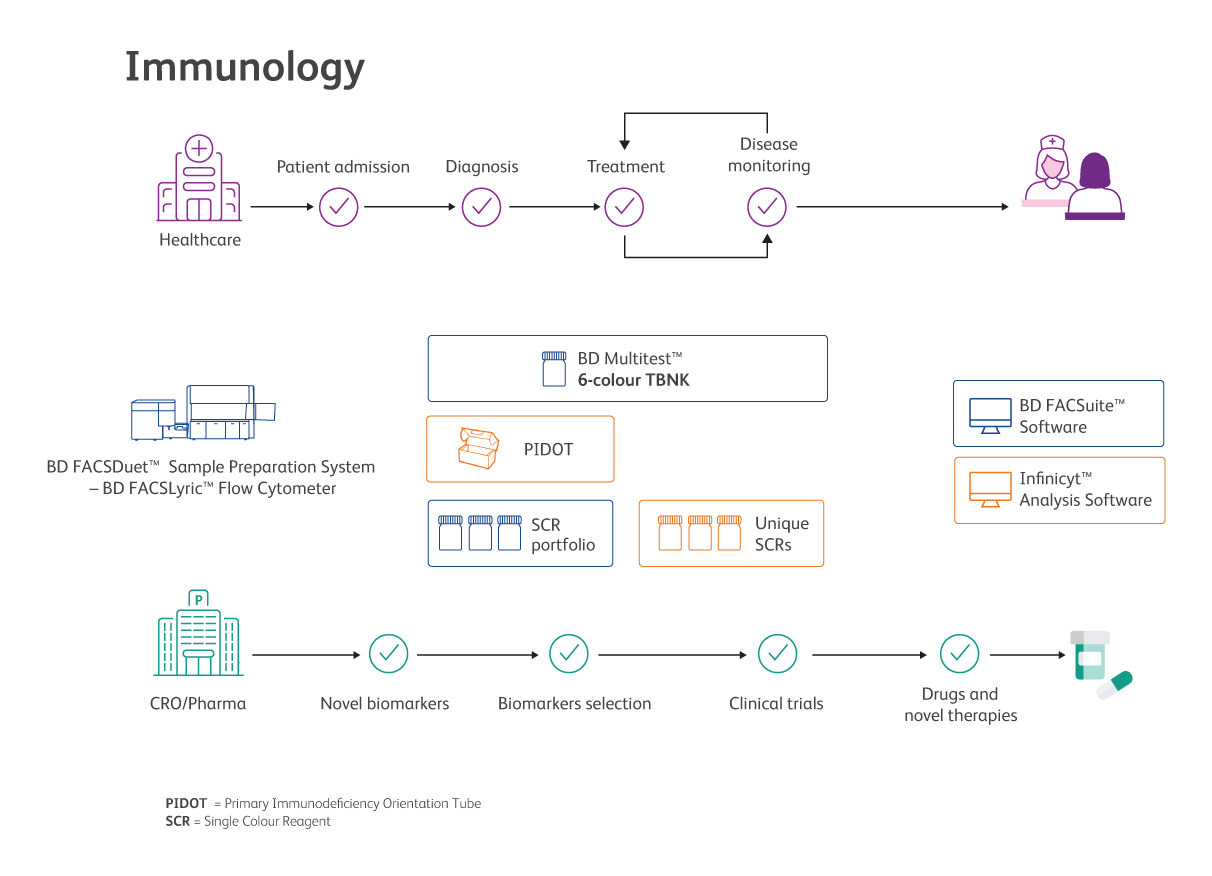
The synergy of Cytognos and BD for Immunology
Immunology at Cytognos
Cytognos offers a highly sensitive method for the screening of suspected primary immunodeficiencies (PIDs) by flow cytometry4.
Most patients with PIDs present common clinical manifestations as the result of immune dysfunction. Cytognos offers solutions aiding to the screening of alterations possibly associated with immunodeficiencies or other immune system disorders, to orient towards diagnosis and further treatment4.
Immunology at BD
BD offers a portfolio for identifying and quantifying T, B and NK lymphocyte subsets for the immunological assessment of normal individuals and patients having, or suspected of having, secondary immune deficiency.
Individuals with human immunodeficiency virus (HIV) typically exhibit a steady decrease in CD3+CD4+ lymphocyte counts as the infection progresses5. While those with COVID-19 disease typically exhibit a decrease in CD3+CD4+ and/or CD3+CD8+ lymphocyte counts with increasing disease severity5.
Discover our new clinical flow cytometry family!
Combined solutions to better serve clinicians and their patients in the fields of onco-haematology and immunology.
Talk to a representative if you want to know more about Cytognos joining BD
*Required fields
References
- van Dongen JJM, Lhermitte L, Böttcher S, et al. on behalf of the EuroFlow Consortium (EU-FP6, LSHB-CT-2006-018708). EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9): 1908-1975. doi: 10.1038/leu.2012.120
- van der Velden VHJ, Flores-Montero J, Perez-Andres M, et al. Optimization and testing of dried antibody tube: The EuroFlow LST and PIDOT tubes as examples. J Immunol Methods. 2019;475:112287. doi: 10.1016/j.jim.2017.03.011
- Moloney E, Watson H, Barge D, et al. Efficiency and health economic evaluations of BD OneFlow™ Flow Cytometry Reagents for diagnosing chronic lymphoid leukemia. Cytometry B Clin Cytom. 2019;96(6):514-520. doi: 10.1002/cyto.b.21779
- Van der Burg M., Kalina T., Perez-Andres M., et al. on behalf of the EuroFlow PID consortium. The EuroFlow PID Orientation Tube for Flow Cytometric Diagnostic Screening of Primary Immunodeficiencies of the Lymphoid System. Front Immunol. 2019; 10:246. doi:10.3389/fimmu.2019.00246
- BD Multitest™ 6-Color TBNK Reagent Instructions for Use. v08; 2020.
![]()
BD FACSDuet™ Sample Preparation System and BD FACSLyric™ Flow Cytometer with the BD FACSuite™ Clinical and BD FACSuite™ applications are CE marked in compliance with the European In Vitro Diagnostic Medical Device Regulation (EU) 2017/746.
BD Multitest™ 6- Colour TBNK is CE marked in compliance with the European In Vitro Diagnostic Medical Device Regulation (EU) 2017/746.
BD Flow Cytometers and BD FACSDuet™ Sample Preparation System are Class 1 Laser Products.
The EuroFlow trademark is the property of the EuroFlow Consortium and cannot be reproduced or published without prior written permission from the EuroFlow coordinator (www.euroflow.org).
Cytognos and Infinicyt are trademarks or registered trademarks of Cytognos, S.L.
