-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




Flow cytometric analysis of FoxP3 expressed in human lymphocytes. Human peripheral blood mononuclear cells (PBMC) were stained with PerCP-Cy5.5 anti-human CD4 (clone L200, Cat. No. 552838) and PE anti-human CD25 (Clone 2A3, Cat. No. 341009) simultaneously. The PBMC were fixed and permeabilized (see Recommended Assay Procedure) followed by intracellular staining with BD Horizon™ V450 Mouse anti-Human FoxP3 (clone 236A/E7; Cat No. 561182). Two-color flow cytometric dot plots were derived from gated events based on the light scattering characteristics of lymphocytes and fluorescence characteristics of CD4+ or CD25+, respectively, shown as either FoxP3 versus CD25 (left panel) or CD4 versus FoxP3 (right panel). Flow cytometry was performed on a BD LSRII™ Flow Cytometer System.


BD Horizon™ V450 Mouse anti-Human FoxP3

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Cell Preparation and Staining Procedures for Fluorochrome-Conjugated Anti-Human FoxP3 Antibody
1. Bring the buffers to room temperature (RT) before use. Prepare working solutions of the BD Pharmingen Human FoxP3 Buffer Set
Cat. No. 560098 (For the buffer preparation, please see the Technical Data Sheet of Cat. No. 560098 buffer instructions for details).
2. Prepare human PBMC. Suspend the cells with BD Pharmingen™ Stain Buffer (FBS)* to ten million cells/ml.
3. Pipette appropriate amount of surface staining reagent to the bottom of each 12 x 75 mm tube.
4. Add 100 µl of cells per tube, vortex, incubate for 20 minutes at RT protected from light.
5. Add 2 ml of wash buffer. Centrifuge 250 x g for 10 minutes to pellet the cells and remove wash buffer.
6. To fix the cells, gently resuspend the cell pellet in residual volume of wash buffer and then add 2 ml of 1x Human FoxP3 Buffer A.
Vortex. Incubate for 10 minutes at RT in the dark.
7. Centrifuge 500 x g for 5 minutes, and remove fixative. Caution: Be aware the pellet is buoyant.
8. To wash cells, resuspend each cell pellet in 2 ml of BD Pharmingen Stain Buffer (FBS)*, and centrifuge 500 x g for 5 minutes. Remove
wash buffer.
9. To permeabilize the cells, gently resuspend pellet in residual volume of wash buffer and then add 0.5 ml of 1x working solution
Human FoxP3 Buffer C to each tube. Vortex. Incubate for 30 minutes at RT protected from light.
10. To wash cells, add 2 ml of BD Pharmingen Stain Buffer (FBS)* to each tube, centrifuge 500 x g for 5 minutes at RT. Remove buffer
and repeat wash step. Remove buffer.
11. Add conjugated FoxP3 antibody at appropriate concentrations to resuspend the pellet. Gently shake or vortex.
12. Incubate for 30 minutes in the dark at RT.
13. Repeat wash step #10.
14. Resuspend in wash buffer and analyze immediately.
Optional Add 300 µl of 1% formaldehyde in 1x PBS and store at 4°C. Analyze cells within 24 hours.
* We recommend using the BD Pharmingen™ Stain Buffer (FBS; Cat No. 554656) for all wash steps and covering tubes during incubation steps with caps or parafilm. We also recommend optimizing forward scatter and side scatter voltages to visualize lymphocytes as separate from debris, red cell ghosts and/or platelets before acquisition.
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- An isotype control should be used at the same concentration as the antibody of interest.
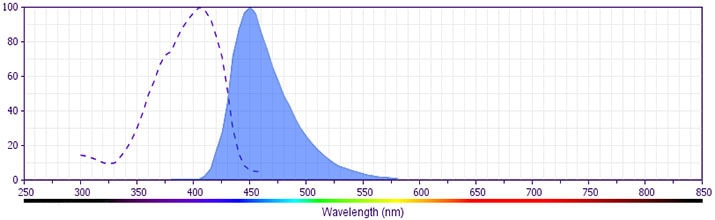
- BD Horizon V450 has a maximum absorption of 406 nm and maximum emission of 450 nm. Before staining with this reagent, please confirm that your flow cytometer is capable of exciting the fluorochrome and discriminating the resulting fluorescence.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Pacific Blue™ is a trademark of Molecular Probes, Inc., Eugene, OR.
Companion Products


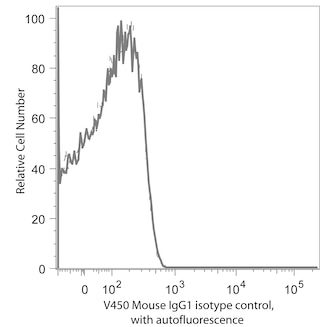
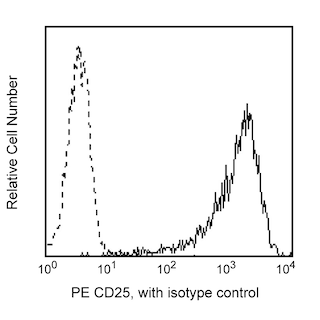
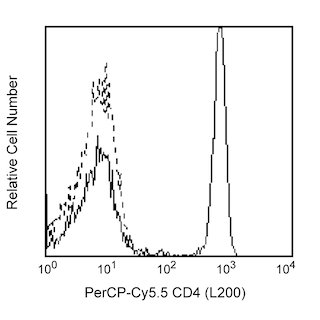

The 236A/E7 antibody reacts with the human FoxP3 (Forkhead box protein P3) transcription factor, a member of the forkhead or winged helix family of transcription factors. The expression of FoxP3, also known as Scurfin, IPEX and JM2, has been found to be associated with CD4+ CD25+ regulatory T cells and represents a specific marker for these cells. Flow cytometric analysis has shown that FoxP3 is expressed by the majority of CD4+ CD25+ (high) T cells in peripheral blood while less than half of CD4+ CD25+ (intermediate) cell population are FoxP3 positive. Approximately 5-10% of peripheral CD4+ cells are CD4+ CD25+ T regulatory cells. T regulatory cells are thought to play a critical role in the control of T cell mediated autoimmunity by suppressing the proliferation and cytokine production of other T cells. To support this hypothesis, it has been found that Foxp3 is mutated in scurfy (sf) mice. Cumulative evidence suggests that the 236A/E7 antibody recognizes epitopes from both isoforms of Exon 2 alternatively-spliced variants and is located in the region between Exon 2 and the Zn/LZ domains (aa 105-235).
The antibody is conjugated to BD Horizon™ V450, which has been developed for use in multicolor flow cytometry experiments and is available exclusively from BD Biosciences. It is excited by the Violet laser Ex max of 406 nm and has an Em Max at 450 nm. Conjugates with BD Horizon™ V450 can be used in place of Pacific Blue™ conjugates.
Development References (8)
-
Alvaro T, Lejeune M, Salvado MT, et al. Outcome in Hodgkin's lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005; 11(4):1467-1473. (Clone-specific: Immunohistochemistry). View Reference
-
Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001; 27(1):68-73. (Biology). View Reference
-
Fox BC, Bignone PA, Brown PJ, Banham AH. Defense of the clone: antibody 259D effectively labels human FOXP3 in a variety of applications. Blood. 2008; 111(7):3897-3899. (Clone-specific: Western blot). View Reference
-
Lennon G, Auffray C, Polymeropoulos M, Soares MB. Consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996; 33(1):151-152. (Biology). View Reference
-
Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005; 35(6):1681-1691. (Immunogen: Flow cytometry). View Reference
-
Roncador G, Garcia JF, Maestre L, et al. FOXP3, a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia. 2005; 19(12):2247-2253. (Clone-specific: Immunohistochemistry). View Reference
-
Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001; 27(1):18-20. (Biology). View Reference
-
Wolf D, Wolf AM, Rumpold H, et al. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005; 11(23):8326-8331. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.