-
Your selected country is
Middle East / Africa
- Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




Two-color flow cytometric analysis of IL-10 expression in stimulated human lymphocytes. An enriched population of CD4+ peripheral blood mononuclear cells (PBMC) was obtained by panning using plate-bound Purified NA/LE Mouse Anti-Human CD4 antibody (Cat. No. 555343). The CD4+ PBMC were cultured (5 d) with plate-bound NA/LE Mouse Anti-Human CD3 (Cat. No. 555329; 10 μg/ml, coated overnight at 4°C) and soluble NA/LE Mouse Anti-Human CD28 (Cat. No. 555725; 1 μg/ml) antibodies plus recombinant Human IL-2 (Cat. No. 554603; 20 ng/ml) and IL-4 (Cat. No. 554605; 40 ng/ml) proteins. The cells were restimulated (5 h) with Phorbol 12-Myristate 13-Acetate (PMA; Sigma P8139; 50 ng/ml) and ionomycin (Sigma I9657; 1 μg/ml) in the presence of BD GolgiStop™ Protein Transport Inhibitor (Cat. No. 554724). After harvesting, the cells were fixed using BD Cytofix™ Fixation Buffer (Cat. No. 554655) and permeabilized with BD Perm/Wash™ Buffer (Cat. No. 554723). The cells were stained in BD Perm/Wash™ Buffer with BD Horizon™ BV421 Mouse Anti- Human CD4 antibody (Cat. No. 565997/566907), and with either PerCP-Cy5.5 Rat IgG2a, κ Isotype Control (Cat. No. 550765; Left Plot) or PerCP-Cy5.5 Mouse Anti-Human IL-10 antibody (Cat. No. 567409/567414; Right Plot). A bivariate pseudocolor density plot showing the coexpressed levels of IL-10 (or Ig Isotype control staining) versus CD4 was derived from gated events with the forward and side light-scatter characteristics of intact lymphocytes. Flow cytometry and data analysis were performed using a BD LSRFortessa™ Cell Analyzer System and FlowJo™ software.


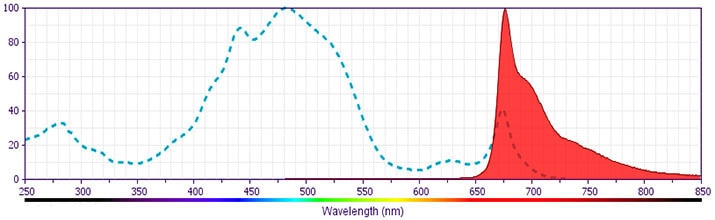
BD Pharmingen™ PerCP-Cy5.5 Rat Anti-Human IL-10

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
BD® CompBeads can be used as surrogates to assess fluorescence spillover (Compensation). When fluorochrome conjugated antibodies are bound to BD® CompBeads, they have spectral properties very similar to cells. However, for some fluorochromes there can be small differences in spectral emissions compared to cells, resulting in spillover values that differ when compared to biological controls. It is strongly recommended that when using a reagent for the first time, users compare the spillover on cells and BD CompBeads to ensure that BD® CompBeads are appropriate for your specific cellular application.
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Please observe the following precautions: Absorption of visible light can significantly alter the energy transfer occurring in any tandem fluorochrome conjugate; therefore, we recommend that special precautions be taken (such as wrapping vials, tubes, or racks in aluminum foil) to prevent exposure of conjugated reagents, including cells stained with those reagents, to room illumination.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- This reagent has been pre-diluted for use at the recommended Volume per Test. We typically use 1 × 10^6 cells in a 100-µl experimental sample (a test).
- PerCP-Cy5.5–labelled antibodies can be used with FITC- and R-PE–labelled reagents in single-laser flow cytometers with no significant spectral overlap of PerCP-Cy5.5, FITC, and R-PE fluorescence.
- PerCP-Cy5.5 is optimized for use with a single argon ion laser emitting 488-nm light. Because of the broad absorption spectrum of the tandem fluorochrome, extra care must be taken when using dual-laser cytometers, which may directly excite both PerCP and Cy5.5™. We recommend the use of cross-beam compensation during data acquisition or software compensation during data analysis.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- An isotype control should be used at the same concentration as the antibody of interest.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
- Cy is a trademark of Global Life Sciences Solutions Germany GmbH or an affiliate doing business as Cytiva.
Companion Products






The JES3-19F1 monoclonal antibody specifically recognizes human Interleukin-10 (IL-10) that is encoded by IL10. IL-10 is also known as Cytokine Synthesis Inhibitory Factor (CSIF), B cell-derived T cell growth factor (B-TCGF), and T-cell growth inhibitory factor (TGIF). The JES3-19F1 antibody crossreacts with ebvIL-10 protein, the Epstein-Barr viral IL-10 homolog (viral IL-10 or vIL-10) encoded by the BCRF1 gene. IL-10 is produced by a variety of cells such as some activated T cells and B cells including regulatory T cells (Treg) and B cells (Breg), monocytes and macrophages, dendritic cells (DC), keratinocytes, and mast cells. IL-10 is a multifunctional cytokine that can downregulate immune and proinflammatory responses. IL-10 can act to reduce expression of major histocompatibility complex class II antigens, costimulatory molecules, or proinflammatory cytokines including IL-1β, IL-2, IL-3, IL-12, IFN-γ, TNF or GM-CSF expressed by activated monocytes, macrophages, dendritic cells (DC), natural killer (NK) cells, or T cells. IL-10 has been shown to play a role in chronic viral infections. IL-10 can also enhance B cell survival, proliferation, and differentiation to become antibody-producing cells. The JES3-19F1 antibody reportedly neutralizes the biological activity of human IL-10 and ebvIL-10. IL-10 mediates its biological activities by signaling through a heterotetrameric receptor complex composed of the type II cytokine receptor subunits CD210a (IL-10 Rα) and CD210b (IL-10 Rβ).
Development References (8)
-
Andersson J, Abrams J, Bjork L, et al. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994; 83(1):16-24. (Clone-specific: Immunohistochemistry). View Reference
-
Brodeur ND, Spencer JV. Antibodies to human IL-10 neutralize ebvIL-10-mediated cytokine suppression but have no effect on cmvIL-10 activity.. Virus Res. 2010; 153(2):265-8. (Clone-specific: Bioassay, Blocking, ELISA, Functional assay, Inhibition, Neutralization, Western blot). View Reference
-
Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines.. J Immunol. 2002; 169(5):2498-506. (Clone-specific: Flow cytometry). View Reference
-
Mielle J, Audo R, Hahne M, et al. IL-10 Producing B Cells Ability to Induce Regulatory T Cells Is Maintained in Rheumatoid Arthritis. Front Immunol. 2018; 9:961. (Clone-specific: Flow cytometry). View Reference
-
Schlaak JF, Schmitt E, Hüls C, Meyer zum Büschenfelde KH, Fleischer B. A sensitive and specific bioassay for the detection of human interleukin-10. J Immunol Methods. 1994; 168(1):49-54. (Clone-specific: Bioassay, Functional assay, Inhibition). View Reference
-
Wehrens EJ, Mijnheer G, Duurland CL, et al. Functional human regulatory T cells fail to control autoimmune inflammation due to PKB/c-akt hyperactivation in effector cells.. Blood. 2011; 118(13):3538-48. (Clone-specific: Flow cytometry). View Reference
-
Yin Y, Mitson-Salazar A, Prussin C. Detection of Intracellular Cytokines by Flow Cytometry. Curr Protoc Immunol. 2015; 110:6.24.21-26.24.18. (Methodology: Flow cytometry). View Reference
-
Zielinski CE, Mele F, Aschenbrenner D, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012; 484(7395):514-518. (Clone-specific: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.