Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Recombinant Human IL-3
(RUO)

Recombinant Human IL-3
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Description
Interleukin-3 (IL-3) is a species specific colony stimulating factor which stimulates colony formation of megakaryocytes, neutrophils, and macrophages from bone marrow cultures. Human IL-3 is a 15 kD protein containing 133 amino acid residues. Recombinant human IL-3 (Cat. No. 554604) is supplied as a frozen liquid comprised of 0.22 µm sterile-filtered aqueous buffered solution containing glycerol and bovine serum albumin, with no preservatives. Recombinant human IL-3 is ≥ 95% pure as determined by SDS-PAGE, and an absorbance assay based on the Beers-Lambert law. The endotoxin level is ≤ 0.1 ng per μg of human IL-3, as measured in a chromogenic LAL assay.
Preparation And Storage
Recommended Assay Procedures
Upon initial thawing, recombinant human IL-3 (Cat. No. 554604) should be aliquoted into polypropylene microtubes and frozen at -80°C for future use. Alternatively, the product can be diluted in sterile neutral buffer containing not less than 0.5 - 1.0 mg/mL carrier protein, such as human or bovine serum albumin, aliquoted and stored at -80°C. Failure to add carrier protein or store at indicated temperatures may result in a loss of activity. Carrier proteins should be pre-screened for possible effects in each investigator's experimental system. Carrier proteins may have an undesired influence on experimental results due to toxicity, high endotoxin levels or possible blocking activity.
ELISA Standard: Recombinant human IL-3 (Cat. No. 554604) is useful as a quantitative standard for measuring human IL-3 protein levels using sandwich ELISA with purified BVD8-3G11 (Cat. No. 554672) as a capture antibody and biotinylated BVD3-1F9 (Cat. No. 554674) as the detection antibody. To obtain linear standard curves, investigators may want to consider using doubling dilutions of recombinant human IL-3 from 2000 - 15 pg/mL to be included in each ELISA plate.
Bioassay: Investigators are advised that the Bioassay application is not routinely tested for this material and are highly encouraged to both titrate this material and include appropriate controls in relevant experiments. An activity range of 0.3 - 1.4 x 10^8 units/mg, encompassing an
ED50= 70 - 300 pg/mL, has previously been reported using TF-1 as indicator cells for proliferation, with a unit defined as the amount of material needed to stimulate a half-maximal response at cytokine saturation.
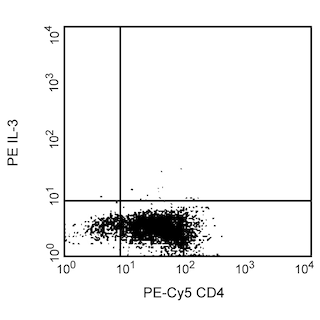
Blocking: Recombinant human IL-3 (Cat. No 554604) can be used as a blocking control for flow cytometric analysis when used with PE-conjugated BVD3-1F9 antibody (Cat. No. 554676). Investigators are advised that the blocking application is not routinely tested for this material. Intracellular cytokine staining techniques and the use of blocking controls are described in detail by C.Prussin and D.Metcalfe.
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products




Development References (3)
-
Frendl G. Interleukin 3: from colony-stimulating factor to pluripotent immunoregulatory cytokine. Int J Immunopharmacol. 1992; 14:421-430. (Biology). View Reference
-
Kitamura T, Tange T, Terasawa T, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989; 140(2):323-334. (Biology). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.