Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?




Two-color flow cytometric analysis of IFN-γ expression by stimulated mouse splenocytes. Mouse splenic leucocytes were stimulated for 5 hours with Phorbol 12-Myristate 13-Acetate (PMA; Sigma P-8139; 50 ng/ml) and Ionomycin (Sigma I-0634; 1 μg/ml) in the presence of BD GolgiStop™ Protein Transport Inhibitor (containing Monensin) (Cat. No. 554724). The cells were harvested, washed with BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656), and fixed with BD Cytofix™ Fixation Buffer (Cat. No. 554655). The cells were then washed and stained in BD Perm/Wash™ Buffer (Cat. No. 554723) with Alexa Fluor® 488 Rat Anti-Mouse CD4 antibody (Cat. No. 557667) and either BD Horizon™ BV480 Rat IgG1, κ Isotype Control (Cat. No. 566098; Left Plot) or BD Horizon BV480 Rat Anti-Mouse IFN-γ antibody (Cat. No. 566097/566151; Right Plot) by using the BD Biosciences Intracellular Cytokine Staining protocol. Two-color flow cytometric contour plots showing the correlated expression of IFN-γ (or Ig Isotype control staining) versus CD4 were derived from gated events with the forward and side light-scatter characteristics of intact stimulated leucocytes. Flow cytometric analysis was performed using a BD™ LSR II Flow Cytometer System.


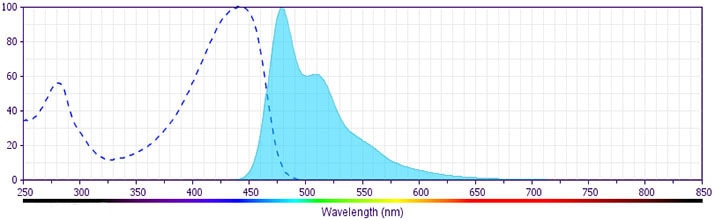
BD Horizon™ BV480 Rat Anti-Mouse IFN-γ

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant™ Stain Buffer should be used anytime BD Horizon Brilliant™ dyes are used in a multicolor flow cytometry panel. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. When BD Horizon Brilliant Stain Buffer is used in in the multicolor panel, it should also be used in the corresponding compensation controls for all dyes to achieve the most accurate compensation. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794/566349) or the BD Horizon Brilliant Stain Buffer Plus (Cat. No. 566385).
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- BD Horizon Brilliant Violet 480 is covered by one or more of the following US patents: 8,575,303; 8,354,239.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products






The XMG1.2 monoclonal antibody specifically binds to mouse interferon-γ (IFN-γ) protein. IFN-γ is a pleiotropic cytokine, of approximately 15-17 kDa, involved in the regulation of inflammatory and immune responses. It plays an important role in activation, growth, and differentiation of T and B lymphocytes, macrophages, NK cells and other non-hematopoietic cell types. IFN-γ production is associated with the Th1 cell differentiation. The purified form of this antibody has been reported to be a neutralizing antibody.
Development References (7)
-
Abrams JS, Roncarolo MG, Yssel H, Andersson U, Gleich GJ, Silver JE. Strategies of anti-cytokine monoclonal antibody development: immunoassay of IL-10 and IL-5 in clinical samples. Immunol Rev. 1992; 127:5-24. (Clone-specific: ELISA, Neutralization). View Reference
-
Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987; 166(5):1229-1244. (Clone-specific: ELISA). View Reference
-
Klinman D and Nutman T. ELISPOT assay to detect cytokine-secreting murine and human cells. In: Coligan J, Kruisbeek A, Margulies D, Shevach E, Strober W, ed. Current Protocols in Immunology. 1994:6-19.
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology). View Reference
-
Sander B, Hoiden I, Andersson U, Moller E, Abrams JS. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and spleen. Cytokine detection by immunoassay and intracellular immunostaining. J Immunol Methods. 1993; 166(2):201-214. (Clone-specific). View Reference
-
Suzuki Y, Yang Q, Conley FK, Abrams JS, Remington JS. Antibody against interleukin-6 reduces inflammation and numbers of cysts in brains of mice with toxoplasmic encephalitis. Infect Immun. 1994; 62(7):2773-2778. (Clone-specific: In vivo exacerbation, Neutralization). View Reference
-
Yang X, HayGlass KT. A simple, sensitive, dual mAb based ELISA for murine gamma interferon determination: comparison with two common bioassays. J Immunoassay. 1993; 14(3):129-148. (Clone-specific: ELISA). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.