Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


APC Mouse Anti-Human CD11b
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
The monoclonal antibody is supplied as 100 µg purified immunoglobulin in 2.0 mL (50 µg/mL) of phosphate-buffered saline (PBS). The PE conjugate is supplied as 100 µg in 2.0 mL (50 µg/mL). The APC conjugate is supplied as 25 µg in 0.5 mL (50 µg/mL). PBS contains gelatin and 0.1% sodium azide.
Store vials at 2° to 8°C. Conjugated forms should not be frozen and should be protected from prolonged exposure to light. Each reagent is stable for the period shown on the bottle label when stored as directed.
The CD11b antibody, clone D12, is derived from the hybridization of NS-1 mouse myeloma cells with spleen cells isolated from BALB/c mice immunized with peripheral blood T lymphocytes. The CD11b antibody recognizes a human leucocyte antigen that is the C3bi complement receptor (CR3). CD11b is specific for the 165-kilodalton (kDa) α subunit of the CD11b/CD18 antigen heterodimer. The 95-kDa CD18 antigen, the β chain of leucocyte function–associated antigen-1 (LFA-1β), is common to the CD11a/CD18 (LFA-1) and CD11c/CD18 antigen heterodimers.
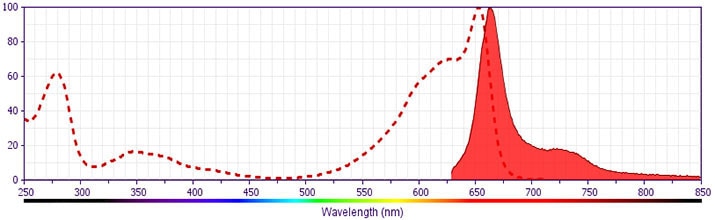
Development References (10)
-
Lanier LL, Phillips JH. A map of the cell surface antigens expressed on resting and activated human natural killer cells. In: Reinherz EL. Ellis L. Reinherz .. et al., ed. Leukocyte typing II. New York: Springer-Verlag; 1986:157-170.
-
Bernstein ID, Self S. Joint report of the Myeloid Section of the Second International Workshop on Human Leukocyte Differentiation Antigens. In: Reinherz EL, Haynes BF, Nadler LM, Bernstein ID, ed. Leukocyte Typing II: Human Myeloid and Hematopoietic Cells. New York, NY: Springer-Verlag; 1986:1-25.
-
Bray RA, Gottschalk LR, Landay AL, Gebel HM. Differential surface marker expression in patients with CD-16+ lymphoproliferative disorders: in vivo model for NK differentiation.. Hum Immunol. 1987; 19(2):105-15. (Biology). View Reference
-
Gebel HM, Kaizer H, Landay AL. Characterization of circulating suppressor T lymphocytes in bone marrow transplant recipients.. Transplantation. 1987; 43(2):258-63. (Biology). View Reference
-
Landay A, Gartland GL, Clement LT. Characterization of a phenotypically distinct subpopulation of Leu-2+ cells that suppresses T cell proliferative responses.. J Immunol. 1983; 131(6):2757-61. (Biology). View Reference
-
Landay A, Gartland GL, Clement LT. Characterization of a phenotypically distinct subpopulation of Leu-2+ cells that suppresses T cell proliferative responses.. J Immunol. 1983; 131(6):2757-61. (Biology). View Reference
-
Patarroyo M, Makgoba MW. Leucocyte adhesion to cells. Molecular basis, physiological relevance, and abnormalities.. Scand J Immunol. 1989; 30(2):129-64. (Biology). View Reference
-
Repo H, Jansson SE, Leirisalo-Repo M. Anticoagulant selection influences flow cytometric determination of CD11b upregulation in vivo and ex vivo.. J Immunol Methods. 1995; 185(1):65-79. (Biology). View Reference
-
Ross GD, Cain JA, Lachmann PJ. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b.. J Immunol. 1985; 134(5):3307-15. (Biology). View Reference
-
Shalekoff S, Page-Shipp L, Tiemessen CT. Effects of anticoagulants and temperature on expression of activation markers CD11b and HLA-DR on human leukocytes.. Clin Diagn Lab Immunol. 1998; 5(5):695-702. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Although not required, these products are manufactured in accordance with Good Manufacturing Practices.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.