-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- BD® OMICS-One Immune Profiler Protein Panel
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Training
- Flow Cytometry Basic Training
-
Product-Based Training
- FACSAria Product Based Training
- FACSMelody Product-Based Training
- FACSLyric Product-Based Training
- FACSCanto Product-Based Training
- LSRFortessa Product-Based Training
- FACSymphony Product-Based Training
- FACSDuet Product-Based Training
- HTS Product-Based Training
- BD FACSDiscover™ S8 Cell Sorter Product Training
-
Advanced Training
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Transplantation
Stem cell transplantation is performed after high-dose chemotherapy (HDC) to restore a cancer patient’s blood and immune cell production capacity. Every year, >30,000 patients with blood-related malignancies receive HDC, which, if the response is satisfactory, could subsequently be followed by hematopoietic stem cell transplantation (HSCT).1 Improvements in transplantation techniques, including the wider use of cell selection, have contributed to a significant reduction in the morbidity and mortality associated with conventional transplantation. HDC with transplant is now standard therapy in multiple myeloma, acute myeloid leukemia in first remission, and intermediate-grade non-Hodgkin's lymphoma.
Bone marrow versus peripheral blood for stem cell transplantation
Hematopoietic stem cells (HSC) from the bone marrow can be induced to move to the peripheral blood as a response to growth factors or chemotherapy; this is used in both autologous and allogenic HSCT.2 HSCs are primarily found in bone marrow niches, but a small fraction of HSCs can also be found in the peripheral blood.3 Following exogenous stimulation, such as chemotherapy or using growth factors such as granulocyte colony stimulating factor (G-CSF) and filgrastim, the number of HSCs in the peripheral blood increases, either becoming on par or even exceeding the number in the bone marrow. This pharmacologically induced egress of HSCs into peripheral blood, called mobilization, is utilized as the preferred strategy for generating HSCs for transplantation.3 Autologous stem cell transplantation almost exclusively uses peripheral blood, while for allogeneic SCT, T-lymphocytes in the peripheral blood could pose some danger.2 The number of viable CD45+/CD34+ cells will determine the quality of specimen for transplantation.
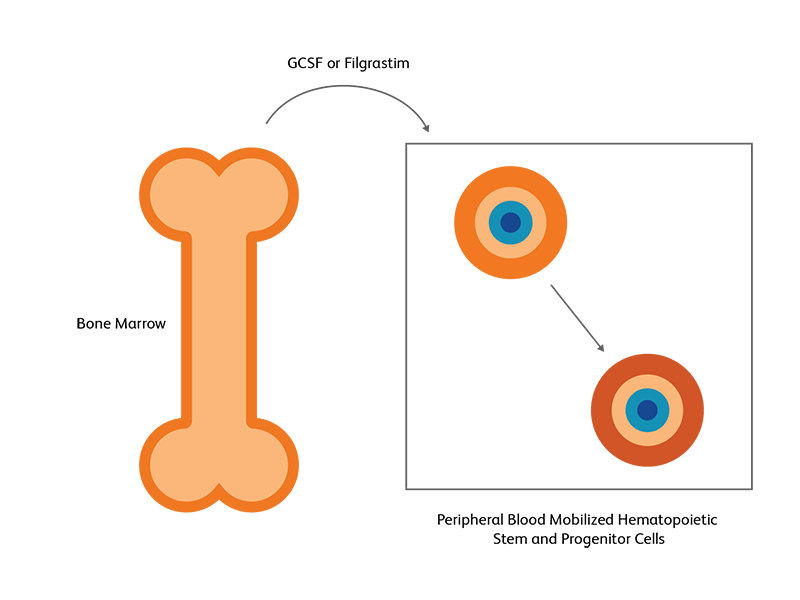
CD34 as the marker of hematopoietic stem cells
CD34, a transmembrane phosphoglycoprotein, is present on immature hematopoietic precursor cells and all hematopoietic colony-forming cells in bone marrow and blood, including unipotent and pluripotent progenitor cells.4 CD34 expression is historically related to hematopoietic cells and it is considered as the marker of HSCs. For many hematopoietic malignancies, collection and infusion of CD34+ hematopoietic stem/progenitor cells following chemotherapy is critical. G-CSF is a commonly used mobilizing agent for practically all autologous and a majority of allogeneic HSCTs and is known to increase CD34+ concentration.
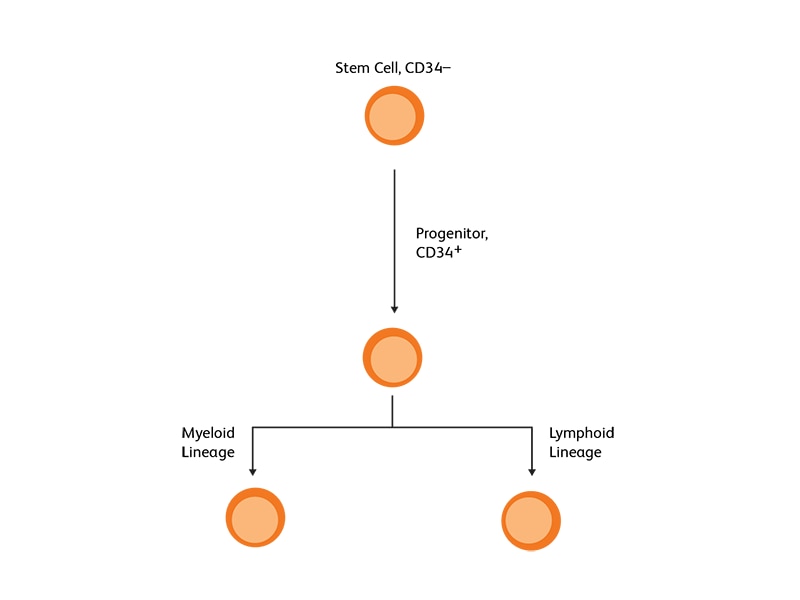
Measurement and selection of CD34
An accurate measurement of CD34 is critical for dose requirement protocols in stem cell transplantation.5 An incorrectly high result could lead to an infusate with less than the recommended threshold dose of CD34+ cells. Quantitating the CD34+ cell population can also be useful during mobilization as well as for determining the optimal timing of apheresis sessions to make sure that enough CD34+ cells have been harvested. Flow cytometric enumeration of CD34+ HSCs and progenitor cells is an established method for the evaluation of bone marrow and stem cell grafts.6 Earlier flow cytometry methods, such as the Milan/Mulhouse and International Society of Hematotherapy and Graft Engineering (ISHAGE) protocols,7 were two-platform methods, using flow cytometry as well as a second platform such as an automated hematology analyzer. The more recent single-platform methods exclusively use a flow cytometer to determine absolute CD34+ counts.6 Fluorochrome-conjugated monoclonal antibodies directed against CD34 molecule can be used to identify CD34+ cells by flow cytometry. Flow cytometric applications for CD34+ cell identification and enumeration provide a rapid, quantitative and reproducible method to evaluate the progenitor cell population. Development of the standardized ISHAGE protocol7 that considers the structural characteristics of the CD34 molecule and the epitopes detected by CD34 monoclonal antibodies further aids in flow cytometry–based measurement of CD34+ markers.
BD Biosciences offers flow cytometers and assays for the enumeration of CD34+ cells.
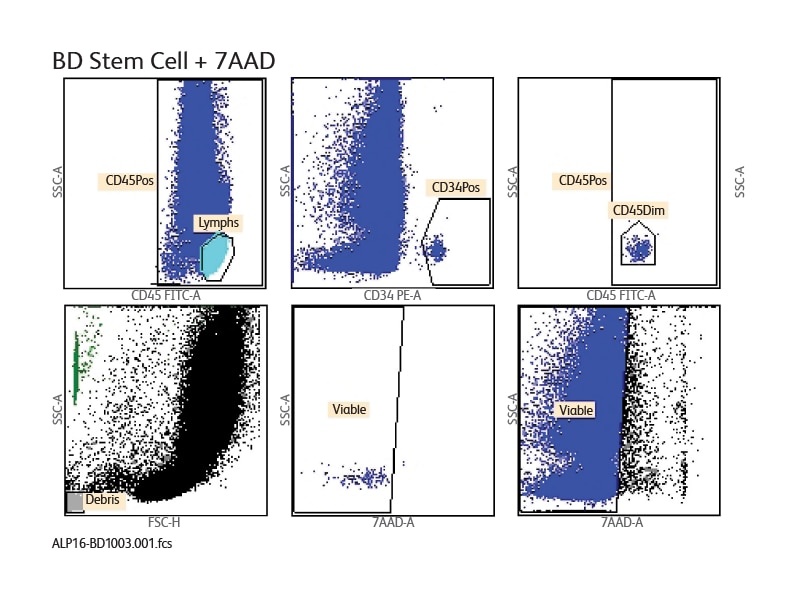
The BD® Stem Cell Enumeration (SCE) Assay enumerates total viable CD34+ and CD45+ cells as a percentage of total viable leukocytes. It incorporates BD Trucount™ Technology for an accurate and reproducible single-platform technology.7 Enumeration of the cell populations in this assay is obtained using either an automated or a manual method for gating and analysis.
The single-tube assay is performed by staining the sample with the reagent in individual BD Trucount™ Tubes for absolute counts. When a sample is added to the reagent, the fluorochrome-labeled antibodies in the reagent bind specifically to the cell surface. Additionally, the lyophilized pellet in the BD Trucount™ Tube dissolves, releasing a known number of fluorescent beads. The 7-AAD dye is added to assess viability of the cells. Cells that are 7-AAD+ are not viable. Ammonium chloride is added to lyse erythrocytes before the sample is acquired on a flow cytometer. During analysis of the sample, the concentration of viable CD34+ cells and viable CD45+ cells, and the percentage of viable CD34+ cells in the viable CD45+ cell population, are calculated.
Sample data generated using the BD® Stem Cell Enumeration Assay
CD34+ viable absolute counts were enumerated and percentages of CD34+ cells were determined with the BD® Stem Cell Enumeration (SCE) Kit on the BD FACSCanto™ Flow Cytometer. A sample lab report of the BD® Stem Cell Enumeration Kit on the BD FACSCanto™ II Flow Cytometer is shown. A lab report is generated for each sample and displays plots, analyzed data and QC messages.
The BD® Cell Stem Enumeration Kit available for use with the BD FACSCanto™ System combines the precision of the BD Trucount™ Absolute Counting Beads with ISHAGE-based protocol guidelines for standardized and accurate results.
References
- Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10(2):120-136. doi: 10.1016/j.stem.2012.01.006
- Jansen J, Hanks S, Thompson JM, Dugan MJ, Akard LP. Transplantation of hematopoietic stem cells from the peripheral blood. J Cell Mol Med. 2005;9(1):37-50. doi: 10.1111/j.1582-4934.2005.tb00335.x
- Karpova D, Rettig MP, DiPersio JF. Mobilized peripheral blood: an updated perspective. F1000Res. 2019;8:F1000 Faculty Rev-2125. doi: 10.12688/f1000research.21129.1x
- Schlossman SF, ed. Leucocyte Typing V: White Cell Differentiation Antigens. Oxford University Press; 1995. 1:840-846.
- Langermayer I, Weaver C, Buckner CD, et al. Engraftment of patients with lymphoid malignancies transplanted with autologous bone marrow, peripheral blood stem cells or both. Bone Marrow Transplant. 1995;15(2):241-246.
- Gajakowska A, Oldak T, Jastrzewska M, et al. Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells in leukapheresis product and bone marrow for clinical transplantation: a comparison of three methods. Folia Histochem Cytobiol. 2006;44(1):53-60.
- Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J Hematother. 1996;5(3):213-226. doi: 10.1089/scd.1.1996.5.213
The BD® Stem Cell Enumeration (SCE) Kit provides simultaneous enumeration of viable dual-positive CD45+/CD34+ hematopoietic stem cell populations in CD34+ absolute counts (cells/μL) as well as the percentage of the total viable leukocyte count that is CD34+ (%CD34). The following specimens can be analyzed with this kit: normal and mobilized peripheral blood, fresh and thawed leukopheresis products, fresh and thawed bone marrow, and fresh and thawed cord blood. The kit is intended for in vitro diagnostic (IVD) use on either a BD FACSCalibur™ Flow Cytometer using BD CellQuest™ or BD CellQuest™ Pro Software or a BD FACSCanto™ II Flow Cytometer using BD FACSCanto™ Clinical Software.
BD flow cytometers are Class 1 Laser Products.
The products shown here may not be approved for sale in Singapore yet. Should you require further information please contact BDB Singapore Marketing.