Products
-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
Solutions
Discover & Learn
-
Thought Leadership
- News
- Blogs
-
Scientific Publications
-
Events
- Expanding PARADIGM to Infectious Disease Modeling: HIV & Tuberculosis
- CYTO 2023: Advancing the World of Cytometry
- Advances in Immune Monitoring Series
- Validating Flow Cytometry Assays for Cell Therapy
- Enhancing Cell Analysis with a New Set of Eyes
- BD Biosciences at International Clinical Cytometry Society 2025
Resources & Tools
Support
You are now leaving the BD Biosciences website. The site you are about to visit is operated by a third party. The link to this site neither makes nor implies any representation or warranty for any products or services offered on a third-party site and is intended only to enable convenient access to the third-party site and for no other purpose. Do you want to continue?
Old Browser
For the best web browsing experience, please use Chrome, Safari or Firefox, minimum versions 77.0.3865, 12.1.2 and 68, respectively.
Please Note
This page has been recently translated and is available in French now.
Please confirm your location
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?
Intracellular Staining
Intracellular Staining of Human Red Blood Cells
- For each sample to be stained, fix 10 µl of whole blood in 1 ml cold 0.05% glutaraldehyde for 10 minutes at room temperature. Vortex after addition of cells.
- Wash once with 5 ml staining buffer (PBS with 1%FBS, 0.09%NaN 3). Centrifuge at 200 x g for 5 minutes.
- Resuspend the cell pellet by vortexing in 0.5 ml 0.1% Triton X-100. Incubate for 10 minutes at room temperature.
- Pellet by centrifugation at 200 x g for 5 minutes and aspirate the supernatant.
- Resuspend the red blood cell pellet in 0.5 ml staining buffer.
- Into new tubes, add primary antibody to appropriate concentration in staining buffer to make 100 µl total volume. For example, if the antibody test size volume is 20 µl, add this amount to 80 µl of staining buffer. Add 10 µl of red blood cell suspension to each tube. Incubate for 20 minutes at room temperature (in the dark).
- Wash with 2 ml of staining buffer at 200 x g for 5 minutes.
- For purified and biotin conjugated antibodies: add 50 µl of diluted secondary reagent, incubate at RT for 15-20 minutes. For direct conjugates proceed to step 10.
- Repeat step 7.
- Add 0.5 ml of staining buffer to each tube.
- Proceed to flow cytometric analysis.
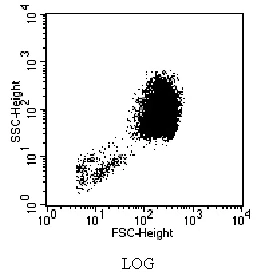
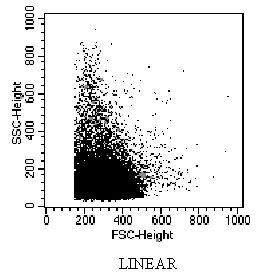
Data Acquisition Note: Setting FSC and SSC detectors to log mode provides better resolution compared to linear mode.