-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® OMICS-One Immune Profiler Protein Panel
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
Thought Leadership
- News
- Blogs
-
Scientific Publications
-
Events
- Expanding PARADIGM to Infectious Disease Modeling: HIV & Tuberculosis
- CYTO 2023: Advancing the World of Cytometry
- Advances in Immune Monitoring Series
- Validating Flow Cytometry Assays for Cell Therapy
- Enhancing Cell Analysis with a New Set of Eyes
- BD Biosciences at International Clinical Cytometry Society 2025
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current location site or be switched to your location?

Rheumatology Research
Rheumatic diseases, such as rheumatoid arthritis (RA), lupus and multiple sclerosis, are caused by autoimmune responses. While in immune deficiency disorders the immune system fails to elicit the appropriate immune responses, in autoimmune disorders the immune system overresponds against one’s own antigens. This failure to distinguish between self and nonself antigens arises from breach of immune tolerance, the preventative mechanisms the immune system has in place to prevent attacking itself.1 Autoimmunity could affect specific tissues or it could be systemic (lupus). Some diseases such as ankylosing spondylosis are considered both autoimmune as well as inflammatory arthritic diseases.
Examples of rheumatic diseases investigated in clinical research
Rheumatoid arthritis (RA) is an autoimmune disorder characterized by inflammation and damage of the joints throughout the body, including hands and feet and affects about 0.5–1% of the population, being more common among women than men in the United States.2
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disease. It affects multiple organ systems, such as the skin, kidneys, lungs and the central nervous system, by producing autoantibodies.3
Ankylosing spondylosis (AS) is a chronic, progressive inflammatory rheumatic disease of the axial musculoskeletal system caused by multiple genes.4
Rheumatoid Arthritis
Types of RA
RA could be divided into two categories:
- Anti-citrullination protein antibodies (ACPA) positive
- ACPA negative
During inflammation, the amino acid arginine is converted into citrulline by peptidylarginine deaminase (PAD). This process, called citrullination, elicits formation of antibodies, which are almost certain indicators of development of RA within a few years.5 The ACAP positive type is more aggressive than the ACPA negative type.6
Immunological progression of RA
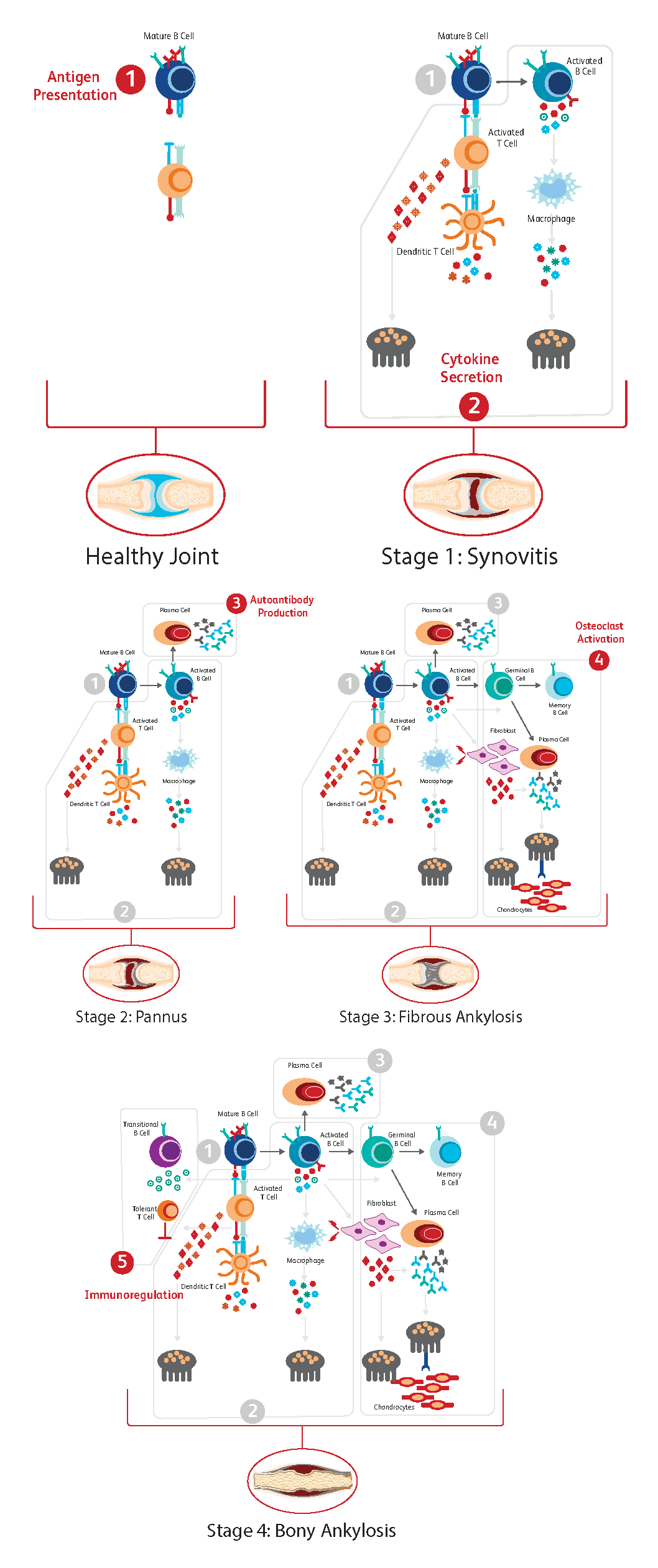
The progression of RA occurs gradually, closely following a concatenation of immunological events.7 The process has been divided into five steps as described below:
- Antigen Presentation
Synovial inflammation is driven by infiltration of CD4 T-cells and macrophages. Mature B-cells and dendritic cells present antigens to T-cells, which leads to T-cell activation and their differentiation into proinflammatory cytokine-producing effector T-cells, which in turn stimulates macrophage cytokine production.
- Cytokine Secretion
Cytokine secretion is the next step in the process, with different immune cells secreting specific cytokines.
B-cells: interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α and IL-17
T-cells: IL-17 and TNF-α
Macrophages: IL-1, IL-6 and (TNF)-α
Fibroblasts and T-cells: receptor activator of nuclear factor ligand (RANKL)
Together these cytokines stimulate the expression of TRANCE/RANKL that is essential for osteoclast differentiation.
- Autoantibody Production
B-T cell interactions result in activation and differentiation of plasma cells responsible for autoantibody production and secretion. Autoantibodies to IgG are produced (for example, rheumatoid factor (RF) and ACPAs).
- Osteoclast Activation
Autoantibodies, cytokines and RANKL stimulate osteoclasts, which lead to bone resorption and chondrocyte-driven cartilage damage. Osteoclast abundance and accelerated bone damage is further driven by B-cells as plasma cell-derived autoantibodies able to recognize citrullinated vimentin promote the differentiation of monocytes to osteoclasts.
- Immunoregulation
In contrast to activated B-cells, transitional B-cells can inhibit osteoclast formation in an immunoregulatory fashion through the provision of IL-10 and other mechanisms.
Genetic basis of RA, SLE and AS
Genetic factors contribute significantly to RA. In particular, the human leukocyte antigen (HLA) locus accounts for about 50% of genetic predisposition to RA, with a strong involvement of HLA-DRB1, a major histocompatibility complex (MHC) class II molecule, with several HLA-DRB1 alleles implicated across different ethnic populations.6 Other non-HLA susceptibility genes, such as protein-arginine deiminase type 4 (PADI4) and IL-2 receptor subunit α, are also implicated in RA.
SLE is a complex genetic disease with the involvement of multiple genes. HLA class II DRB is also implicated in SLE.3,8
HLA-B27, an MHC class I molecule, has a strong association with AS. HLA-B27 testing is routinely used to screen for AS.4
BD Biosciences research tools for RA
The dried, unit-sized, preformulated and optimized BD Horizon™ Dri Treg Panel contains markers used for the characterization of FoxP3+ naïve, translational and effector Treg subsets, directly relevant for autoimmune research.
The BD Horizon™ Dri TBNK + CD20 Panel enables efficient characterization of T, B and NK cells.
The BD Horizon™ Dyes and Antibodies are ideal for characterizing immune cells that have few receptors on the surface and their brightness makes it easy to distinguish these dim cells from others in a sample.
References
- Wang L, Wang F, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369-395. doi: 10.1111/joim.12395
- Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551-1557. doi: 10.1007/s00296-017-3726-1
- Baechler EC, Batliwalla FM, Karypis G, et al. Interferon-inducible gene expression signature in peripheral blood cells patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610-2615. doi: 10.1073/pnas.0337679100
- Dakwar E, Reddy J, Vale FL, Uribe JS. A review of the pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E2. doi: 10.3171/FOC/2008/24/1/E2
- Van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, et al. Autoantibodies to cyclic citrullinated peptides predict progression of rheumatoid arthritis in patients with undifferentiated arthritis: A prospective cohort study. Arthritis Rheum. 2004;50:709-715. doi: 10.1002/art.20044
- Chung IM, Ketharnathan S, Thiruvengadam M, Rajakumar G. Rheumatoid arthritis: the stride from research to clinical practice. Int J Mol Sci. 2016;17(6):900. doi: 10.3390/ijms17060900
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nature Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1
- Parodis I, Stockfelt M, Sjowall C. B cell therapy in systemic lupus erythematosus: From rationale to clinical practice. Front Med (Lausanne). 2020;7:316. doi: 10.3389/fmed.2020.00316
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
The information provided herein is not meant to be used, nor should it be used, to diagnose or treat any medical condition. All content, including text, graphics, images and information etc., contained in or available through this literature is for general information purposes only. For diagnosis or treatment of any medical condition, please consult your physician/doctor. Becton Dickinson India Private Limited and or its affiliates, its employees are not liable for any damages/claims to any person in any manner whatsoever.