Old Browser
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Product Notices
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- An isotype control should be used at the same concentration as the antibody of interest.
- Species cross-reactivity detected in product development may not have been confirmed on every format and/or application.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Companion Products
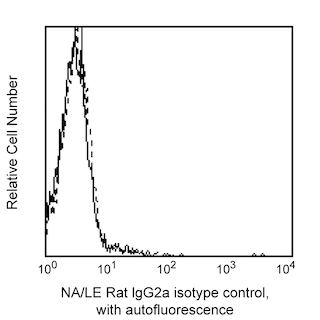
The MQ1-17H12 monoclonal antibody specifically binds to the multifunctional cytokine, human Interleukin-2 (IL-2). IL-2 is produced by activated T cells and has multiple functions that can affect the growth, proliferation, differentiation and survival of many different target cell types including T cells, B cells, NK cells, monocytes and macrophages. The immunogen used to generate the MQ1-17H12 hybridoma was purified recombinant human IL-2 protein. The MQ1-17H12 antibody reportedly neutralizes the biological activity of human IL-2.
Development References (4)
-
Andersson J, Abrams J, Bjork L, et al. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994; 83(1):16-24. (Immunogen). View Reference
-
Fernandez V, Andersson J, Andersson U, Troye-Blomberg M. Cytokine synthesis analyzed at the single-cell level before and after revaccination with tetanus toxoid. Eur J Immunol. 1994; 24(8):1808-1815. (Immunogen). View Reference
-
Gillis S, Ferm MM, Ou W, Smith KA. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978; 120(6):2027-2032. (Biology). View Reference
-
Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Methods. 1995; 188(1):117-128. (Methodology: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Refer to manufacturer's instructions for use and related User Manuals and Technical Data Sheets before using this product as described.
Comparisons, where applicable, are made against older BD technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.