Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Purified Mouse Anti-Cytochrome c
Clone 6H2.B4 (RUO)

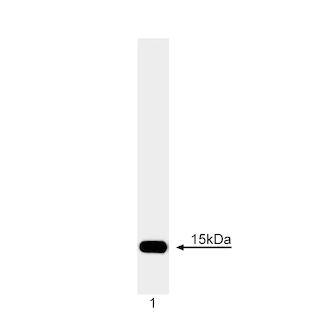
Immunoprecipitation analysis of Cytochrome c. Clone 6H2. B4 (Cat. No. 556432) was used at 2 µg/ml to immunoprecipitate Cytochrome c from P388D1 mouse lymphoma (lane 2), HeLa human carcinoma (lane 3), Jurkat T leukemia (lane 4), and NIH/3T3 mouse fibroblast (lane 5) cell lysates. Cytochrome c was detected by Western blot analysis using clone 7H8.2C12 (Cat. No. 556433) (lanes 1-5). Purified rat Cytochrome c was used as a protein standard in lane 1. Cytochrome c is detected at a molecular weight of ~15 kDa. The upper bands in lane 1 represent dimers or multimers of purified Cytochrome C.

Immunofluorescent staining of HeLa (ATCC CCL-2) cells. Cells were seeded in a 96 well imaging plate (Cat. No. 353219) at ~10,000 cells per well. After overnight incubation, cells were stained using the Triton™ X-100 perm protocol and the anti-Cytochrome C antibody. The second-step reagent was Alexa Fluor 488 anti-mouse IgG (Invitrogen). The image was taken on a BD Pathway™ 855 Bioimaging system using a 20x objective. This antibody also stained U-2 OS (ATCC HTB-96) and A549 (ATCC CCL-185) cells and worked with both the Triton™ X-100 and alcohol perm protocols (see Recommended Assay Procedure).



Immunoprecipitation analysis of Cytochrome c. Clone 6H2. B4 (Cat. No. 556432) was used at 2 µg/ml to immunoprecipitate Cytochrome c from P388D1 mouse lymphoma (lane 2), HeLa human carcinoma (lane 3), Jurkat T leukemia (lane 4), and NIH/3T3 mouse fibroblast (lane 5) cell lysates. Cytochrome c was detected by Western blot analysis using clone 7H8.2C12 (Cat. No. 556433) (lanes 1-5). Purified rat Cytochrome c was used as a protein standard in lane 1. Cytochrome c is detected at a molecular weight of ~15 kDa. The upper bands in lane 1 represent dimers or multimers of purified Cytochrome C.
Immunofluorescent staining of HeLa (ATCC CCL-2) cells. Cells were seeded in a 96 well imaging plate (Cat. No. 353219) at ~10,000 cells per well. After overnight incubation, cells were stained using the Triton™ X-100 perm protocol and the anti-Cytochrome C antibody. The second-step reagent was Alexa Fluor 488 anti-mouse IgG (Invitrogen). The image was taken on a BD Pathway™ 855 Bioimaging system using a 20x objective. This antibody also stained U-2 OS (ATCC HTB-96) and A549 (ATCC CCL-185) cells and worked with both the Triton™ X-100 and alcohol perm protocols (see Recommended Assay Procedure).

Immunoprecipitation analysis of Cytochrome c. Clone 6H2. B4 (Cat. No. 556432) was used at 2 µg/ml to immunoprecipitate Cytochrome c from P388D1 mouse lymphoma (lane 2), HeLa human carcinoma (lane 3), Jurkat T leukemia (lane 4), and NIH/3T3 mouse fibroblast (lane 5) cell lysates. Cytochrome c was detected by Western blot analysis using clone 7H8.2C12 (Cat. No. 556433) (lanes 1-5). Purified rat Cytochrome c was used as a protein standard in lane 1. Cytochrome c is detected at a molecular weight of ~15 kDa. The upper bands in lane 1 represent dimers or multimers of purified Cytochrome C.

Immunofluorescent staining of HeLa (ATCC CCL-2) cells. Cells were seeded in a 96 well imaging plate (Cat. No. 353219) at ~10,000 cells per well. After overnight incubation, cells were stained using the Triton™ X-100 perm protocol and the anti-Cytochrome C antibody. The second-step reagent was Alexa Fluor 488 anti-mouse IgG (Invitrogen). The image was taken on a BD Pathway™ 855 Bioimaging system using a 20x objective. This antibody also stained U-2 OS (ATCC HTB-96) and A549 (ATCC CCL-185) cells and worked with both the Triton™ X-100 and alcohol perm protocols (see Recommended Assay Procedure).


BD Pharmingen™ Purified Mouse Anti-Cytochrome c

BD Pharmingen™ Purified Mouse Anti-Cytochrome c

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Bioimaging
1. Seed the cells in appropriate culture medium at ~10,000 cells per well in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219) and culture overnight.
2. Remove the culture medium from the wells, and fix the cells by adding 100 μl of BD Cytofix™ Fixation Buffer (Cat. No. 554655) to each well. Incubate for 10 minutes at room temperature (RT).
3. Remove the fixative from the wells, and permeabilize the cells using either BD Perm Buffer III, 90% methanol, or Triton™ X-100:
a. Add 100 μl of -20°C 90% methanol or Perm Buffer III (Cat. No. 558050) to each well and incubate for 5 minutes at RT.
OR
b. Add 100 μl of 0.1% Triton™ X-100 to each well and incubate for 5 minutes at RT.
4. Remove the permeabilization buffer, and wash the wells twice with 100 μl of 1× PBS.
5. Remove the PBS, and block the cells by adding 100 μl of BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) to each well. Incubate for 30 minutes at RT.
6. Remove the blocking buffer and add 50 μl of the optimally titrated primary antibody (diluted in Stain Buffer) to each well, and incubate for 1 hour at RT.
7. Remove the primary antibody, and wash the wells three times with 100 μl of 1× PBS.
8. Remove the PBS, and add the second step reagent at its optimally titrated concentration in 50 μl to each well, and incubate in the dark for 1 hour at RT.
9. Remove the second step reagent, and wash the wells three times with 100 μl of 1× PBS.
10. Remove the PBS, and counter-stain the nuclei by adding 200 μl per well of 2 μg/ml Hoechst 33342 (e.g., Sigma-Aldrich Cat. No. B2261) in 1× PBS to each well at least 15 minutes before imaging.
11. View and analyze the cells on an appropriate imaging instrument.
Bioimaging: For more detailed information please refer to http://www.bdbiosciences.com/support/resources/protocols/ceritifed_reagents.jsp
Western blot: For more detailed information please refer to http://www.bdbiosciences.com/pharmingen/protocols/Western_Blotting.shtml
Product Notices
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- Since applications vary, each investigator should titrate the reagent to obtain optimal results.
- This antibody has been developed and certified for the bioimaging application. However, a routine bioimaging test is not performed on every lot. Researchers are encouraged to titrate the reagent for optimal performance.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Triton is a trademark of the Dow Chemical Company.
Data Sheets
Companion Products



A cytochrome is an electron-transporting protein that contains a heme prosthetic group. Cytochromes have been known to be essential components of the mitochondrial respiratory chain since 1925. The iron atom of the heme group in cytochromes alternates between a reduced ferrous (+2) state and an oxidized ferric (+3) state during electron transport in oxidative phosphorylation. Cytochromes are classified into four groups (a, b, c and d) according to spectrochemical characteristics, and there are five cytochromes between coenzyme QH2 and O2 in the electron transport chain. Cytochrome c is a water-soluble protein that either promotes cell survival or death, depending upon its intracellular location. In healthy cells, it is a peripheral membrane protein of the mitochondria that transports electrons from the coenzyme QH2 cytochrome c reductase complex to the cytochrome c oxidase complex. When proapoptotic stimuli induce breakdown of the mitochondria, cytochrome c is released to the cytosol where it functions in the activation of caspases that trigger apoptosis.
The 6H2.B4 monoclonal antibody has been reported to recognize the native and not the denatured form of rat, mouse, and human cytochrome c. Furthermore, studies utilizing competitive ELISA indicate that mAb 6H2.B4 binds to a region around residue 62 of rat cytochrome c.
The 6H2.B4 monoclonal antibody is not useful for western blot analysis. For the western blot application, clone 7H8.2C12 (cat.no. 556433) would be recommended. Suggested positive controls for detecting cytochrome c include P388D mouse lymphoma cells (ATCC CCL-46), HeLa human carcinoma cells (ATCC CCL-2), Jurkat T leukemia cells (ATCC TIB-152) and NIH 3T3 mouse fibroblast cells (ATCC CRL-1658). This antibody is routinely tested by immunoprecipitation. Other applications were tested at BD Biosciences Pharmingen during antibody development only or reported in the literature.
Development References (6)
-
Arnoult D, Grodet A, Lee Y-J, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005; 280(42):35742-35750. (Clone-specific: Immunofluorescence).
-
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006; 13:1423-1433. (Biology).
-
Goshorn SC, Retzel E, Jemmerson R. Common structural features among monoclonal antibodies binding the same antigenic region of cytochrome c. J Biol Chem. 1991; 266(4):2134-2142. (Immunogen: ELISA). View Reference
-
Jemmerson R, Johnson JG. different functional boundaries for the major antigenic region of two cytochromes c. Proc Natl Acad Sci U S A. 1991; 88:4428-4432. (Immunogen: ELISA).
-
Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996; 86(1):147-157. (Clone-specific: Immunoprecipitation). View Reference
-
Rossé T, Olivier R, Monney L, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998; 391(6666):496-499. (Clone-specific: Immunofluorescence). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.