Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Pharmingen™ Alexa Fluor® 555 Mouse anti-H2AX (pS139)
Clone N1-431 (RUO)

Immunofluorescent staining of human cell line. HeLa cells (ATCC CCL-2) were seeded in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219) at ~10,000 cells per well. After overnight culture, the cells were exposed to 2400 Joules UV irradiation (right image) or untreated (left image) and then allowed to recover for 30-60 minutes at 37°C. The cells were fixed, permeabilized with cold methanol, and stained with Alexa Fluor® 555 Mouse anti-H2AX (pS139) (pseudo colored red) according to the Recommended Assay Procedure. Cell nuclei were counterstained with Hoechst 33342 (pseudo colored blue). The images were captured on a BD Pathway™ 435 high-content Bioimager system using a 20X objective and merged using BD AttoVision™ software. This antibody also worked with the Saponin and the Triton™ X-100 Perm/Wash protocols (see Recommended Assay Procedure; Bioimaging protocol link).


Immunofluorescent staining of human cell line. HeLa cells (ATCC CCL-2) were seeded in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219) at ~10,000 cells per well. After overnight culture, the cells were exposed to 2400 Joules UV irradiation (right image) or untreated (left image) and then allowed to recover for 30-60 minutes at 37°C. The cells were fixed, permeabilized with cold methanol, and stained with Alexa Fluor® 555 Mouse anti-H2AX (pS139) (pseudo colored red) according to the Recommended Assay Procedure. Cell nuclei were counterstained with Hoechst 33342 (pseudo colored blue). The images were captured on a BD Pathway™ 435 high-content Bioimager system using a 20X objective and merged using BD AttoVision™ software. This antibody also worked with the Saponin and the Triton™ X-100 Perm/Wash protocols (see Recommended Assay Procedure; Bioimaging protocol link).

Immunofluorescent staining of human cell line. HeLa cells (ATCC CCL-2) were seeded in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219) at ~10,000 cells per well. After overnight culture, the cells were exposed to 2400 Joules UV irradiation (right image) or untreated (left image) and then allowed to recover for 30-60 minutes at 37°C. The cells were fixed, permeabilized with cold methanol, and stained with Alexa Fluor® 555 Mouse anti-H2AX (pS139) (pseudo colored red) according to the Recommended Assay Procedure. Cell nuclei were counterstained with Hoechst 33342 (pseudo colored blue). The images were captured on a BD Pathway™ 435 high-content Bioimager system using a 20X objective and merged using BD AttoVision™ software. This antibody also worked with the Saponin and the Triton™ X-100 Perm/Wash protocols (see Recommended Assay Procedure; Bioimaging protocol link).


BD Pharmingen™ Alexa Fluor® 555 Mouse anti-H2AX (pS139)

Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
Recommended Assay Procedure for Bioimaging:
http://www.bdbiosciences.com/support/resources/protocols/ceritifed_reagents.jsp or http://www.bdbiosciences.com/bioimaging/reagents
1. Seed the cells in appropriate culture medium at an appropriate cell density in a BD Falcon™ 96-well Imaging Plate (Cat. No. 353219), and
culture overnight to 48 hours.
2. Remove the culture medium from the wells, and wash (one to two times) with 100 μl of 1× PBS.
3. Fix the cells by adding 100 µl of fresh 3.7% Formaldehyde in PBS or BD Cytofix™ fixation buffer (Cat. No. 554655) to each well and incubating for 10 minutes at room temperature (RT).
4. Remove the fixative from the wells, and wash the wells (one to two times) with 100 μl of 1× PBS.
5. Permeabilize the cells using either cold methanol (a), Triton™ X-100 (b), or Saponin (c):
a. Add 100 µl of -20°C 90% methanol or -20°C BD™ Phosflow Perm Buffer III (Cat. No. 558050) to each well and incubate for 5 minutes at RT.
b. Add 100 µl of 0.1% Triton™ X-100 to each well and incubate for 5 minutes at RT.
c. Add 100 µl of 1× Perm/Wash buffer (Cat. No. 554723) to each well and incubate for 15 to 30 minutes at RT. Continue to use 1× Perm/Wash buffer for all subsequent wash and dilutions steps.
6. Remove the permeabilization buffer from the wells, and wash one to two times with 100 μl of appropriate buffer (either 1× PBS or 1× Perm/Wash buffer, see step 5.c.).
7. Optional blocking step: Remove the wash buffers, and block the cells by adding 100 µl of blocking buffer BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656) or 3% FBS in appropriate dilution buffer to each well and incubating for 15 to 30 minutes at RT.
8. Dilute the antibody to its optimal working concentration in appropriate dilution buffer. Titrate purified (unconjugated) antibodies and second-step reagents to determine the optimal concentration. If using a Bioimaging Certified antibody conjugate, dilute it 1:10.
9. Add 50 µl of diluted antibody per well and incubate for 60 minutes at RT. Incubate in the dark if using fluorescently labeled antibodies.
10. Remove the antibody, and wash the wells three times with 100 μl of wash buffer. An optional detergent wash (100 μl of 0.05% Tween in 1× PBS) can be included prior to the regular wash steps.
11. If the antibody being used is fluorescently labeled, then move to step 12. Otherwise, if using a purified unlabeled antibody, repeat steps 8 to 10 with a fluorescently labeled second-step reagent to detect the purified antibody.
12. After the final wash, counter-stain the nuclei by adding 100 μl of a 2 μg/ml solution of Hoechst 33342 (eg, Sigma-Aldrich Cat. No. B2261) in 1× PBS to each well at least 15 minutes before imaging.
13. View and analyze the cells on an appropriate imaging instrument. Recommended filters for the BD Pathway™ instruments are:
Instrument Excitation Emission Dichroic
BD Pathway 855 548/20 570LP Fura/FITC
BD Pathway 435 543/22 593/40 FF562
Product Notices
- This reagent has been pre-diluted for use at the recommended Volume per Test when following the Recommended Assay Procedure. A Test is typically ~10,000 cells cultured in a well of a 96-well imaging plate.
- The Alexa Fluor®, Pacific Blue™, and Cascade Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, excluding use in combination with microarrays, or as analyte specific reagents. The Alexa Fluor® dyes (except for Alexa Fluor® 430), Pacific Blue™ dye, and Cascade Blue® dye are covered by pending and issued patents.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Alexa Fluor® is a registered trademark of Molecular Probes, Inc., Eugene, OR.
- Triton is a trademark of the Dow Chemical Company.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
Data Sheets
Companion Products


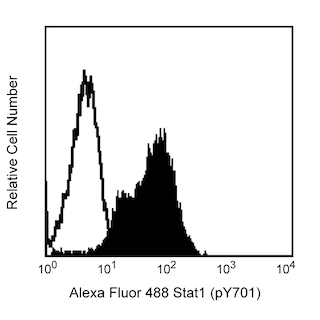

Histones are highly basic proteins that complex with DNA to form chromatin. The H2AX histone (~15 kDa calculated molecular weight) is a member of the H2A histone family whose members are components of nucleosomal histone octamers. Double-stranded breaks in DNA caused by replication errors, apoptosis, or other physiological processes (including, immunoglobulin and TCR gene recombinations) and DNA damage caused by ionizing radiation, UV light, or cytotoxic agents lead to phosphorylation of H2AX on serine 139. H2AX (pS139) is also referred to as H2AX (pS140) when the N-terminal methionine that is normally excised during posttranslational processing is included in amino acid sequence numbering. Kinases such as ataxia telangiectasia mutated (ATM) or ATM-Rad3-related (ATR) phosphorylate H2AX to induce its function. Phosphorylated H2AX (also termed, gamma-H2AX) functions to recruit and localize DNA repair proteins or cell cycle checkpoint factors to the DNA-damaged sites. In this way, phosphorylated H2AX promotes DNA repair and maintains genomic stability and thus helps prevent oncogenic transformations. Immunofluorescent staining and bioimaging analysis of cultured cells can be used to readily identify H2AX (pS139)-containing foci. As such, H2AX (pS139) immunofluorescence localization serves as a biomarker for nuclear sites of DNA damage (e.g., double-stranded DNA breaks) in affected cells.
Development References (5)
-
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001; 276(45):42462-42467. (Biology). View Reference
-
Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst). 2004; 3(8-9):959-967. (Biology). View Reference
-
Kuo LJ, Yang LX. Gamma-H2AX - A novel biomarker for DNA double-strand breaks. In Vivo. 2008; 22(3):305-309. (Biology). View Reference
-
Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000; 275(13):9390-9395. (Biology). View Reference
-
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998; 273(10):5858-5868. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.