Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from {countryName}.
Would you like to stay on the current country site or be switched to your country?


Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Preparation And Storage
Recommended Assay Procedures
For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes (including BD OptiBuild Brilliant reagents) are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794).
Product Notices
- This antibody was developed for use in flow cytometry.
- The production process underwent stringent testing and validation to assure that it generates a high-quality conjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.
- Researchers should determine the optimal concentration of this reagent for their individual applications.
- An isotype control should be used at the same concentration as the antibody of interest.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at www.bdbiosciences.com/colors.
- Please refer to www.bdbiosciences.com/us/s/resources for technical protocols.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- BD Horizon Brilliant Violet 605 is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,227,187; 8,455,613; 8,575,303; 8,354,239.
Companion Products
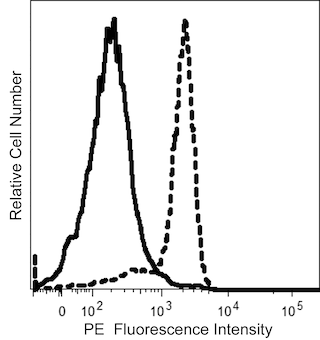




The IA10 monoclonal antibody specifically binds to CD55, which is also known as complement decay-accelerating factor (DAF). CD55 is a glycophosphatidylinositol (GPI)-anchored, single chain membrane glycoprotein of approximately 70 kDa that belongs to the regulators of complement activation (RCA) gene family which includes CD21, CD35, and CD46. CD55 is widely expressed on hematopoietic cells including platelets and erythrocytes, as well as on many non-hematopoietic cells, such as, endothelial cells and epithelial cells. CD55 is involved in protecting cells from damage by autologous activated complement complexes. CD55 prevents the amplification steps of the complement cascade by interfering with the assembly of the C3-convertases, C4b2a and C3bBb, and the C5-convertases, C4b2a3b and C3bBb3b.
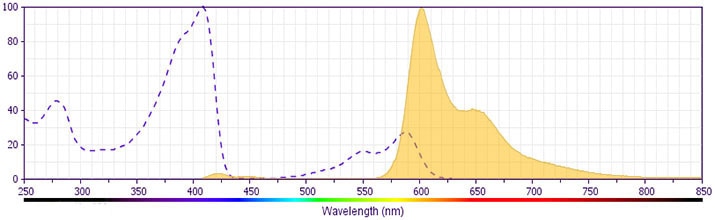
This antibody is conjugated to BD Horizon™ BV605 which is part of the BD Horizon Brilliant™ Violet family of dyes. With an Ex Max of 407-nm and Em Max of 602-nm, BD Horizon BV605 can be excited by a violet laser and detected with a standard 610/20-nm filter set. BD Horizon BV605 is a tandem fluorochrome of BD Horizon BV421 and an acceptor dye with an Em max at 605-nm. Due to the excitation of the acceptor dye by the green (532 nm) and yellow-green (561 nm) lasers, there will be significant spillover into the PE and BD Horizon PE-CF594 detectors off the green or yellow-green lasers. BD Horizon BV605 conjugates are very bright, often exhibiting brightness equivalent to PE conjugates and can be used as a third color off of the violet laser.
Development References (5)
-
Coyne KE, Hall SE, Thompson S, et al. Mapping of epitopes, glycosylation sites, and complement regulatory domains in human decay accelerating factor. J Immunol. 1992; 149(9):2906-2913. (Clone-specific: Blocking, Immunoprecipitation). View Reference
-
Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985; 162(1):75-92. (Immunogen: Blocking, Flow cytometry, Functional assay, Inhibition, Western blot). View Reference
-
Klickstein LB, Springer TA. CD55 cluster report. In: Schlossman SF. Stuart F. Schlossman .. et al., ed. Leucocyte typing V : white cell differentiation antigens : proceedings of the fifth international workshop and conference held in Boston, USA, 3-7 November, 1993. Oxford: Oxford University Press; 1995:1473-1474.
-
Loveland BE, Szokolai K, Johnstone RW, McKenzie IF. Coordinate functions of multiple complement regulating molecules, CD46, CD55, and CD59. Transplant Proc. 1994; 26(3):1070-1071. (Biology). View Reference
-
Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991; 9:431-455. (Biology). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
Report a Site Issue
This form is intended to help us improve our website experience. For other support, please visit our Contact Us page.