-
Reagents
- Flow Cytometry Reagents
-
Western Blotting and Molecular Reagents
- Immunoassay Reagents
-
Single-Cell Multiomics Reagents
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
-
Functional Assays
-
Microscopy and Imaging Reagents
-
Cell Preparation and Separation Reagents
-
- BD® OMICS-Guard Sample Preservation Buffer
- BD® AbSeq Assay
- BD® Single-Cell Multiplexing Kit
- BD Rhapsody™ ATAC-Seq Assays
- BD Rhapsody™ Whole Transcriptome Analysis (WTA) Amplification Kit
- BD Rhapsody™ TCR/BCR Next Multiomic Assays
- BD Rhapsody™ Targeted mRNA Kits
- BD Rhapsody™ Accessory Kits
- BD® OMICS-One Protein Panels
- Norway (English)
-
Change country/language
Old Browser
This page has been recently translated and is available in French now.
Looks like you're visiting us from United States.
Would you like to stay on the current country site or be switched to your country?
BD Horizon™ Brilliant Stain Buffer
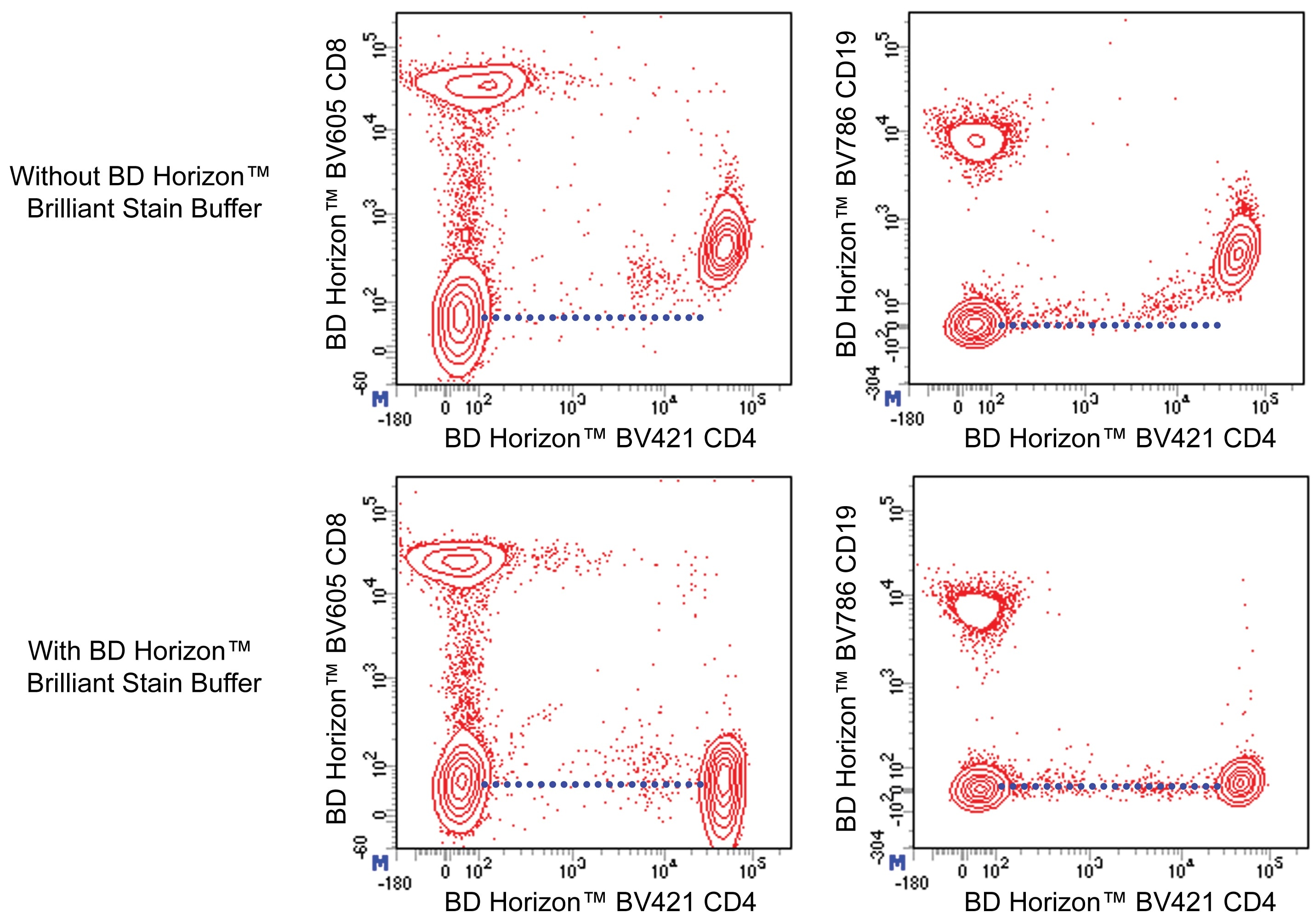
Figure 1. BD Horizon Brilliant Stain Buffer should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant reagents. Whole human blood was stained with BD Horizon™ BV421 Mouse Anti-Human CD4 antibody and either BD Horizon™ BV605 Mouse Anti-Human CD8 antibody (Left Panels) or BD Horizon™ BV786 Mouse Anti-Human CD19 antibody (Right Panel). Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). Two-color flow cytometric dot plots show the correlated expression patterns of CD4 versus either CD8 or CD19 for gated events with the forward and side light-scatter characteristics of viable lymphocytes. Staining cells in the presence of BD Horizon™ Brilliant Stain Buffer (Cat. No. 563794/566349; Lower Panels) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without Brilliant Stain Buffer (Upper Panels). Flow cytometric analysis was performed using a BD™ LSR II Flow Cytometer System.
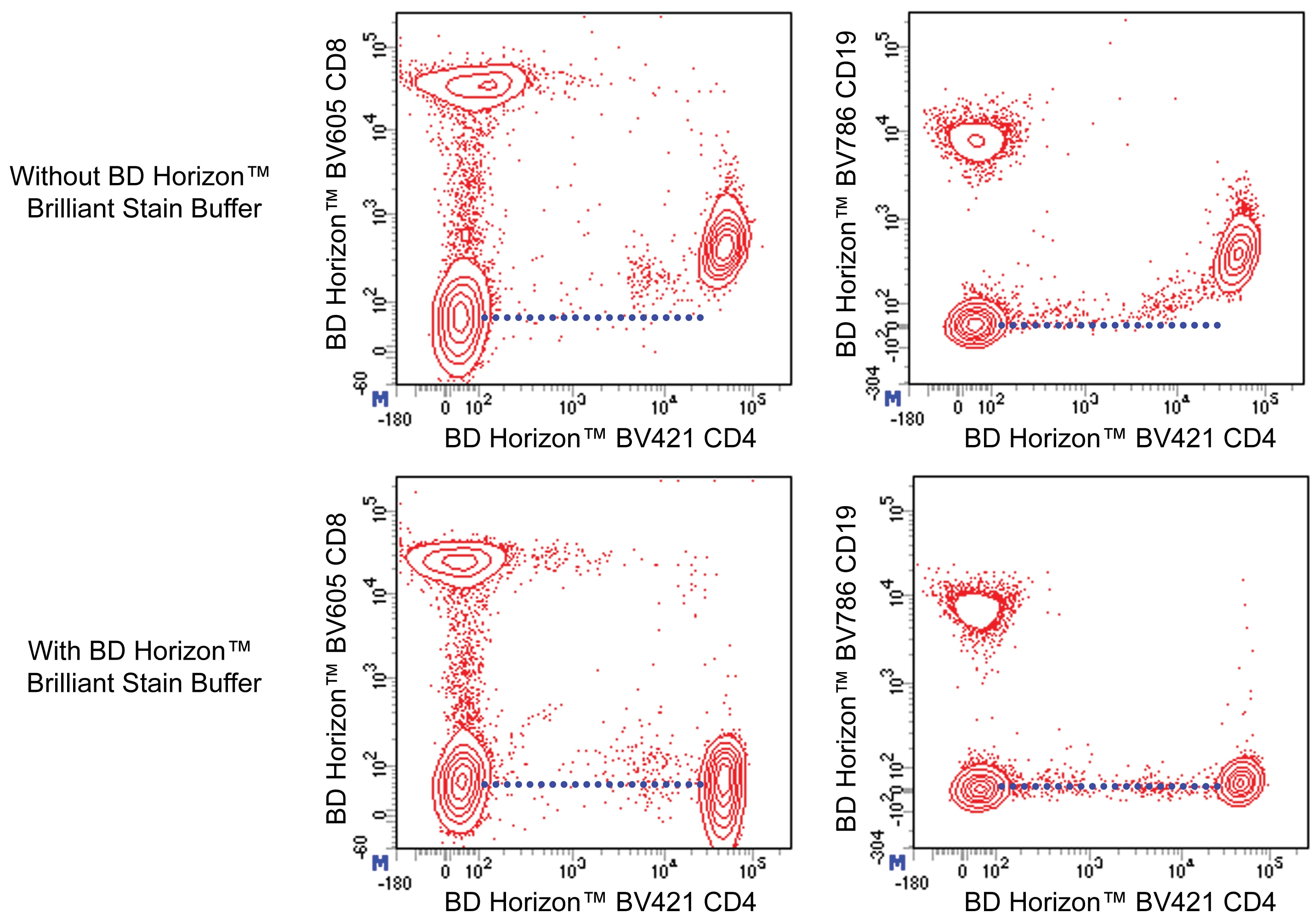


Figure 1. BD Horizon Brilliant Stain Buffer should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant reagents. Whole human blood was stained with BD Horizon™ BV421 Mouse Anti-Human CD4 antibody and either BD Horizon™ BV605 Mouse Anti-Human CD8 antibody (Left Panels) or BD Horizon™ BV786 Mouse Anti-Human CD19 antibody (Right Panel). Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). Two-color flow cytometric dot plots show the correlated expression patterns of CD4 versus either CD8 or CD19 for gated events with the forward and side light-scatter characteristics of viable lymphocytes. Staining cells in the presence of BD Horizon™ Brilliant Stain Buffer (Cat. No. 563794/566349; Lower Panels) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without Brilliant Stain Buffer (Upper Panels). Flow cytometric analysis was performed using a BD™ LSR II Flow Cytometer System.

Figure 1. BD Horizon Brilliant Stain Buffer should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant reagents. Whole human blood was stained with BD Horizon™ BV421 Mouse Anti-Human CD4 antibody and either BD Horizon™ BV605 Mouse Anti-Human CD8 antibody (Left Panels) or BD Horizon™ BV786 Mouse Anti-Human CD19 antibody (Right Panel). Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). Two-color flow cytometric dot plots show the correlated expression patterns of CD4 versus either CD8 or CD19 for gated events with the forward and side light-scatter characteristics of viable lymphocytes. Staining cells in the presence of BD Horizon™ Brilliant Stain Buffer (Cat. No. 563794/566349; Lower Panels) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without Brilliant Stain Buffer (Upper Panels). Flow cytometric analysis was performed using a BD™ LSR II Flow Cytometer System.

Figure 1. BD Horizon Brilliant Stain Buffer should be used in multicolor immunofluorescent staining and flow cytometric analysis when using two or more BD Horizon Brilliant reagents. Whole human blood was stained with BD Horizon™ BV421 Mouse Anti-Human CD4 antibody and either BD Horizon™ BV605 Mouse Anti-Human CD8 antibody (Left Panels) or BD Horizon™ BV786 Mouse Anti-Human CD19 antibody (Right Panel). Erythrocytes were lysed with BD FACS Lysing Solution (Cat. No. 349202). Two-color flow cytometric dot plots show the correlated expression patterns of CD4 versus either CD8 or CD19 for gated events with the forward and side light-scatter characteristics of viable lymphocytes. Staining cells in the presence of BD Horizon™ Brilliant Stain Buffer (Cat. No. 563794/566349; Lower Panels) restores the major lymphocyte populations to their expected fluorescent staining patterns (dotted lines) when compared with cells stained without Brilliant Stain Buffer (Upper Panels). Flow cytometric analysis was performed using a BD™ LSR II Flow Cytometer System.

BD Horizon™ Brilliant Stain Buffer

BD Horizon™ Brilliant Stain Buffer
Regulatory Status Legend
Any use of products other than the permitted use without the express written authorization of Becton, Dickinson and Company is strictly prohibited.
Product Details
Description
The BD Horizon™ Brilliant Stain Buffer is a buffer for the immunofluorescent staining of cells. Brilliant Stain Buffer is a solution that is added to mixtures of certain fluorescent reagents before staining cells. It was designed to complement multicolor flow cytometry experiments that utilize staining reagents conjugated with BD Horizon Brilliant fluorescent polymer dyes. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. For optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime BD Horizon Brilliant dyes are used. The BD Horizon Brilliant Stain Buffer is compatible with the use of other fluorescent staining reagents conjugated with traditional fluorochromes, such as fluorescein, phycoerythrin or Alexa Fluor® dyes.
Preparation And Storage
Recommended Assay Procedures
Protocols for Multicolor Immunofluorescent Staining of Cells Using BD Horizon Brilliant Stain Buffer
Multicolor Staining of Human Cell Samples in Tubes or 96-Well Plates Using Individual Staining Reagents
1. Add 50 μL of BD Horizon Brilliant Stain Buffer to all tubes or desired wells for the experiment
Note: The 50 μL amount of Brilliant Stain Buffer per tube or per well does not depend on the final staining volume or amount of cells used per tube or number of BD fluorescent antibodies used in staining. Although written for use with human cells, these protocols can readily be adapted for analyzing mouse cells or cells from other species, for example, by staining mouse cells at 4°C rather than at room temperature (RT).
2. Add each fluorescent reagent at the recommended volume per test (eg, 5 μL or 20 μL) and then proceed to either Protocol 1, 2, or 3.
Protocol 1 for Staining Whole Blood Samples in Tubes
a. Add 100 μL of human whole blood to each tube
b. Vortex tube contents
c. Incubate (30 min) the suspended cells protected from light at room temperature (RT)
d. Add 2 mL of BD FACS™ Lysing Solution (Cat. No. 349202; 10 min) or BD Pharm Lyse™ Lysing Buffer (Cat. No. 555899; 15 min) per tube and incubate protected from light at RT
e. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
f. Aspirate supernatant; add 2-3 mL of stain/wash buffer, eg, BD Pharmingen™ Stain Buffer (FBS) (Cat. No. 554656)
or BD Pharmingen™ Stain Buffer (BSA) (Cat. No. 554657)
g. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
h. Aspirate the supernatant and resuspend cells in 500 μL of stain/wash buffer for flow cytometric analysis
Protocol 2 for Staining Peripheral Blood Mononuclear Cells or Bulk Erythrocyte-lysed Whole Blood Samples in Tubes
a. Add 100 μL of human cells to each tube
b. Vortex tube contents
c. Incubate (30 min) the suspended cells protected from light at room temperature (RT)
d. Add 2 ml of stain/wash buffer per tube
e. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
f. Aspirate supernatant; add 2-3 mL of stain/wash buffer
g. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
h. Aspirate the supernatant and resuspend cells in 500 μL of stain/wash buffer for flow cytometric analysis
Protocol 3 for Staining Peripheral Blood Mononuclear Cells or Bulk Erythrocyte-lysed Whole Blood Samples in 96-well Plates
Note: When planning for staining in plates, the user must account for the volume of the BD Horizon Brilliant Stain Buffer used. Although written for use with human cells, this 96-well plate-based protocol can readily be adapted for analyzing mouse cells or cells from other species.
a. Add 50 μL of human cells to each well
b. Incubate (30 min) protected from light at RT
c. Wash by adding 100 μL of stain/wash buffer
d. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
e. Aspirate supernatants
f. Resuspend pelleted cells by adding 250 μL of stain/wash buffer
g. Pellet cells by centrifugation (5 min) at 1400-1500 rpm
h. Aspirate supernatants
i. Resuspend pelleted cells thoroughly with 150 μL stain/wash buffer by pipetting the suspended cells several times
j. Transfer well contents to tubes and add additional stain/wash buffer to the tubes as desired for flow cytometric analysis
Note: Alternatively, acquire samples for flow cytometric analysis from the plate directly
Multicolor Staining of Human Cell Samples in Tubes or 96-Well Plates Using Cocktailed Staining Reagents
Instead of adding staining reagents individually to each tube or well of a 96-well plate, it may be desirable to add cocktailed staining reagents, ie, mixtures of two or more fluorescent staining reagents. The following protocol provides an example of how to prepare a "per test" 5-Color Fluorescent Antibody Cocktail that already contains BD Horizon Brilliant Stain Buffer.
Human Samples: Pre-mixed Fluorescent Reagent Cocktails
For each multicolor test of cocktailed fluorescent reagents:
i) Add 50 μL of BD Horizon Brilliant Stain Buffer per test
ii) Add each fluorescent reagent at the recommended volume per test (5 μL or 20 μL)
iii) Mix reagents (especially after adding BD Horizon Brilliant reagents)
iv) Store cocktail at 4°C protected from light if it is to be used later
Note: Protected from light, fluorescent reagent cocktails containing more than one Brilliant Violet and/or Brilliant Blue reagent are best used within 24 hours after preparation when stored at 4ºC or within 4 hours when stored at room temperature. However, when more than one Brilliant Ultraviolet (BUV) reagent is in the cocktail, it is best used within 2 hours after preparation irrespective of storage temperature.
Example of creating a 5-Color Fluorescent Antibody Cocktail containing 2 different Brilliant Violet™ Conjugates
Final Volume per Test = 90 μL
_____________________________________________________________________________________
Total Number of Tests
Volume/Test (μL) 1 3 5 10
Brilliant Stain Buffer 50 50 150 250 500
Reagent 1 (BV) 5 5 15 25 50
Reagent 2 (BV) 5 5 15 25 50
Reagent 3 5 5 15 25 50
Reagent 4 5 5 15 25 50
Reagent 5 20 20 60 100 200
Total Volume 90 90 270 450 900
_____________________________________________________________________________________
Add desired volume of Reagent Cocktail (90 μL in this 5-color example) to all tubes or wells using
the protocols for staining human cells described above.
Compensation and Setup
BD Horizon Brilliant Stain Buffer can be used in single color compensation controls using cells. The buffer is compatible with BD™ Compbeads, however, it has not been tested with compensation beads from other vendors. For the most accurate compensation, compensation controls created with either cells or beads should be exposed to BD Horizon Brilliant Stain Buffer for the same length of time as the corresponding multicolor panel. Additionally, the most accurate compensation will be created when BD Horizon Brilliant Stain Buffer is used in all compensation controls, including Brilliant polymer and non-polymer dyes.
Product Notices
- Alexa Fluor® is a registered trademark of Life Technologies Corporation.
- BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444; 8,575,303; 8,354,239.
- Source of all serum proteins is from USDA inspected abattoirs located in the United States.
- Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in running water before discarding to avoid accumulation of potentially explosive deposits in plumbing.
- Please refer to http://regdocs.bd.com to access safety data sheets (SDS).
Data Sheets
Companion Products



Development References (7)
-
Acosta JR, Douagi I, Andersson DP, et al. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes.. Diabetologia. 2016; 59(3):560-70. (Methodology). View Reference
-
Andrés-Blasco I, Herrero-Cervera A, Vinué Á, et al. Hepatic lipase deficiency produces glucose intolerance, inflammation and hepatic steatosis.. J Endocrinol. 2015; 227(3):179-91. (Methodology). View Reference
-
Ingelman-Sundberg HM, Laestadius Å, Chrapkowska C, et al. Diverse effects on vaccine-specific serum IgG titres and memory B cells upon methotrexate and anti-TNF-α therapy in children with rheumatic diseases: A cross-sectional study.. Vaccine. 2016; 34(10):1304-11. (Methodology: Flow cytometry). View Reference
-
Leijten EF, van Kempen TS, Boes M, et al. Brief report: enrichment of activated group 3 innate lymphoid cells in psoriatic arthritis synovial fluid.. 2015; 67(10):2673-8. (Methodology: Flow cytometry). View Reference
-
McKay FC, Gatt PN, Fewings N, et al. The low EOMES/TBX21 molecular phenotype in multiple sclerosis reflects CD56+ cell dysregulation and is affected by immunomodulatory therapies.. Clin Immunol. 2016; 163:96-107. (Methodology: Flow cytometry). View Reference
-
O'Connor KS, Read SA, Wang M, et al. IFNL3/4 genotype is associated with altered immune cell populations in peripheral blood in chronic hepatitis C infection.. Genes Immun. 2016; 17(6):328-34. (Methodology: Flow cytometry). View Reference
-
Vinué Á, Andrés-Blasco I, Herrero-Cervera A, et al. Ink4/Arf locus restores glucose tolerance and insulin sensitivity by reducing hepatic steatosis and inflammation in mice with impaired IRS2-dependent signalling.. Biochim Biophys Acta. 2015; 1852(9):1729-42. (Methodology: Flow cytometry). View Reference
Please refer to Support Documents for Quality Certificates
Global - Refer to manufacturer's instructions for use and related User Manuals and Technical data sheets before using this products as described
Comparisons, where applicable, are made against older BD Technology, manual methods or are general performance claims. Comparisons are not made against non-BD technologies, unless otherwise noted.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.